Clinical Outcomes
Source: Endocrine Today
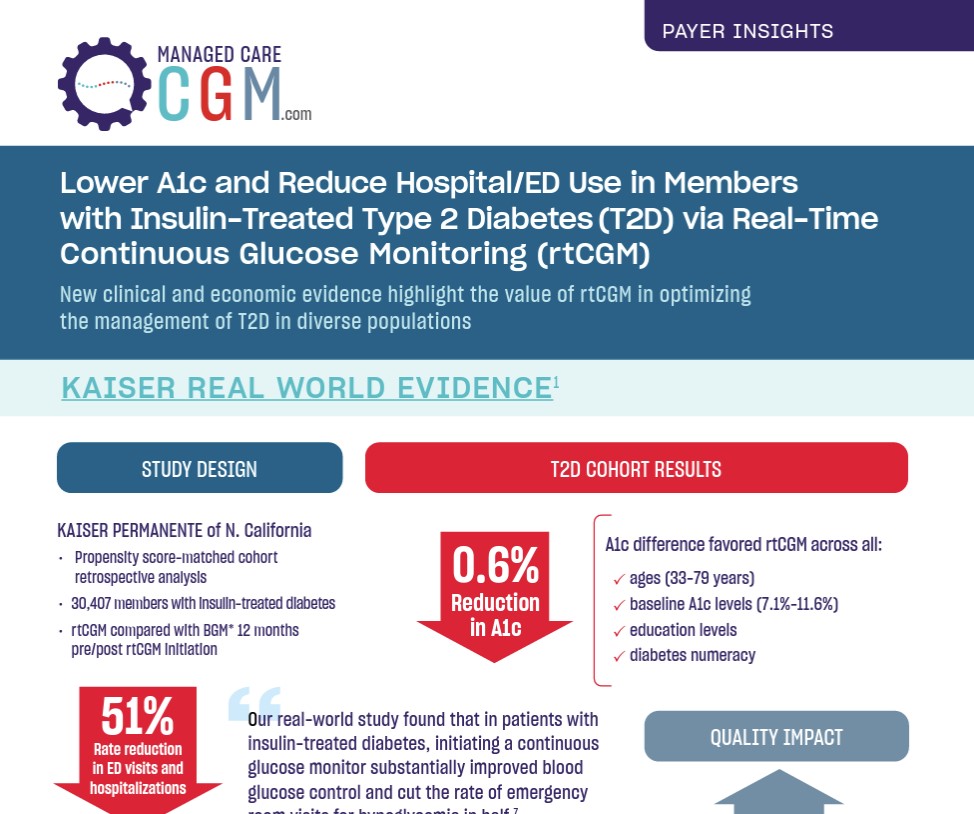
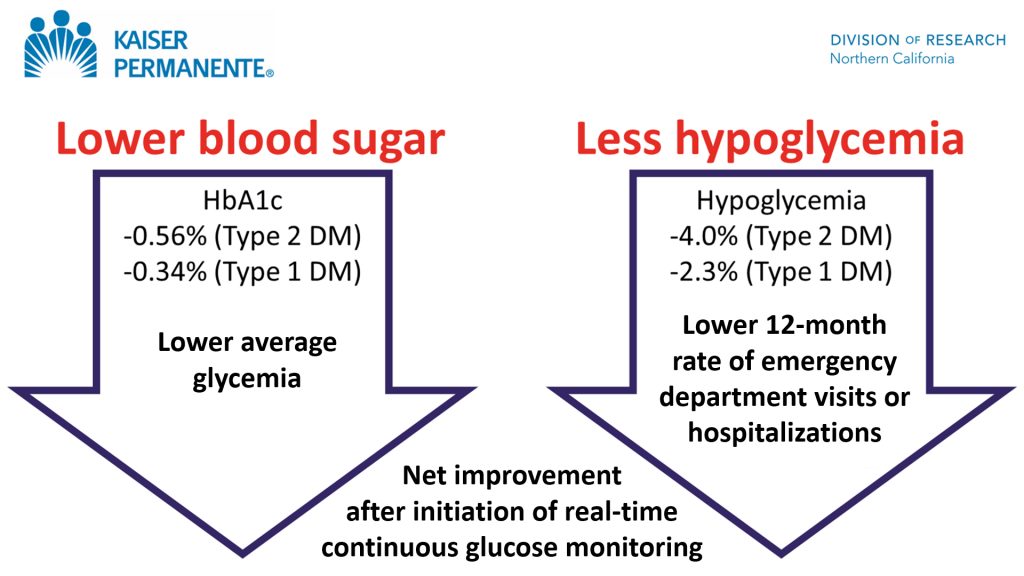
“Our real-world study found that in patients with insulin-treated diabetes, initiating a continuous glucose monitor substantially improved blood glucose control and cut the rate of emergency room visits for hypoglycemia in half,”
Andre J . Karter, PhD
Learn More

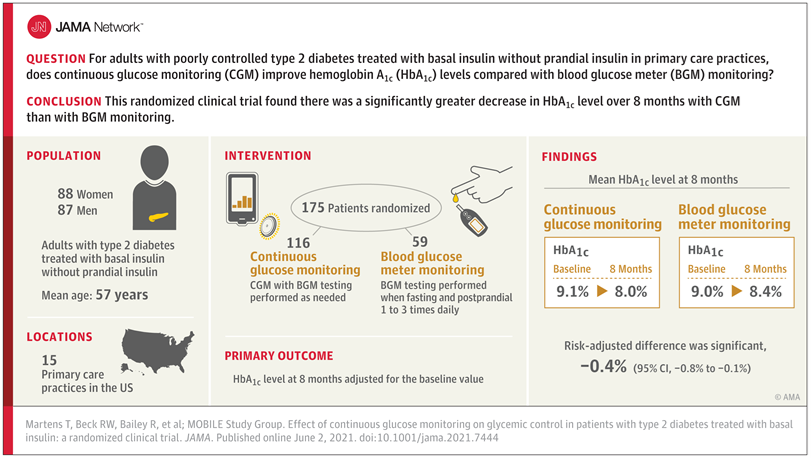
Source: The Journal of the American Medical Association
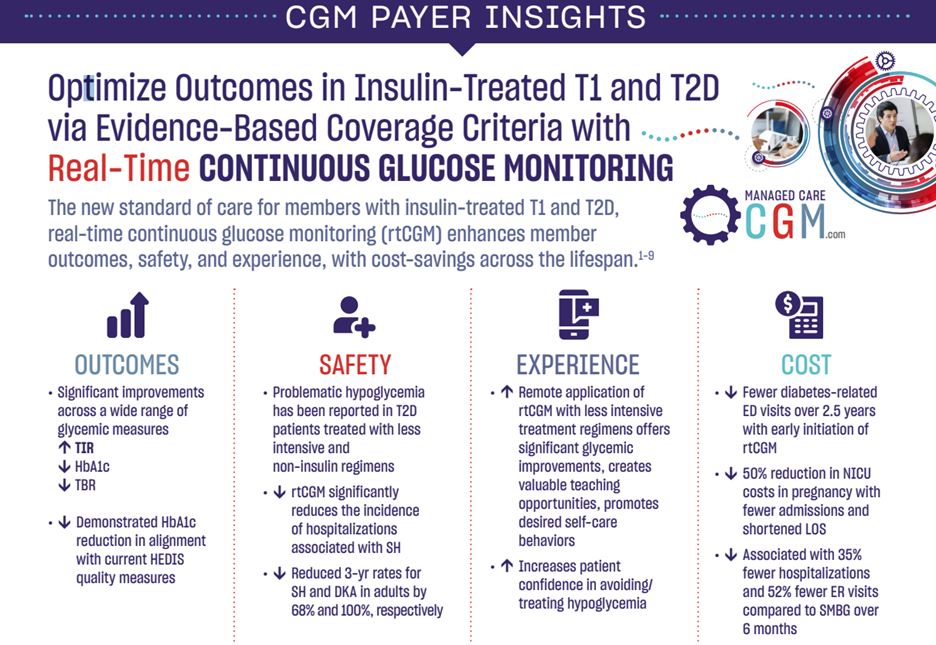
Key Takeaways: Continuous Glucose Monitoring improved outcomes more than intermittent testing of blood glucose in 41,753 patients with insulin-treated diabetes.
“Our real-world study found that in patients with insulin-treated diabetes, initiating a continuous glucose monitor substantially improved blood glucose control and cut the rate of emergency room visits for hypoglycemia in half.”1
Andrew J. Karter, PhD, Senior Research Scientist at Kaiser Permanente Division of Research
1Shaffer R. Real-time CGM lowers HbA1c, reduces ED visits in insulin-treated diabetes. Endocrine Today. June 2021. https://www.healio.com/news/endocrinology/20210607/realtime-cgm-lowers-hba1c-reduces-ed-visits-in-insulintreated-diabetes. Accessed June 24, 2021.
Learn MoreSource: The Journal of the American Medical Association
Authors: Monica E. Peek, MD, MPH, MS; Celeste C. Thomas, MD, MS
“…the studies by Karter et al. and Martens et al. provide additional evidence that patients with type 2 diabetes benefit from the use of CGM in terms of improved HbA1c level, time spent in the target blood glucose range, and reduced hypoglycemic episodes…”
“…institutional changes that promote its use in primary care will go a long way to improving diabetes control and reducing complications, particularly among the populations most in need. The time has come to broaden access to CGM for patients with type 2 diabetes.”
Learn More

Source: The Lancet – June 2021
Key Takeaways:
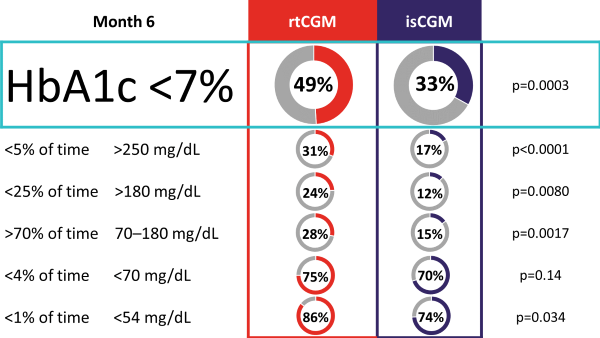
The ALERTT1 trial is the first 6-month, multicenter, prospective, randomized controlled trial comparing rtCGM with isCGM in 254 adults with type 1 diabetes, who previously used isCGM. Mean HbA1c was 7·4% (58 mmol/mol) and a minority of the study population was hypoglycemia unaware (44 [17%] people) or had a history of severe hypoglycemia (29 [11%]). Most (205 [81%]) were treated with multiple daily injections. Findings showed that in an unselected group of people with type 1 diabetes, 6-month use of rtCGM with alert functionality improved time in range (70–180 mg/dL [3.9–10.0 mmol/L]), while HbA1c, time in clinically significant hypoglycemia (< 54 mg/dL [3.0 mmol/L), and hyperglycemia (180 mg/dL [10.0 mmol/L]) were reduced. Additionally, more people on rtCGM achieved glycemic targets as defined by international consensus guidelines, and had less frequently severe hypoglycemia. Moreover, rtCGM users experienced less hypoglycemia worry and higher treatment satisfaction at the end of study.
Percentage of Participants Achieving Consensus Targets
Source: Diabetes Technology and Therapeutics
Key Takeaway: The CONCEPTT (CGM in pregnant women with type 1 diabetes) trial provided high-quality, randomized-controlled trial data demonstrating that the use of real-time CGM was associated with lower HbA1c at 34 weeks, suggesting improved maternal glucose levels during the late second and early third trimesters. Importantly, this was accompanied by 7% higher time in range (TIR) and 5% lower time above range (TAR) without increasing maternal hypoglycemia. Beyond impacting surrogate markers of maternal glycemia, using CGM led to clinically significant reductions in large for gestational-age infants, neonatal hypoglycemia, and neonatal intensive care unit (NICU) admissions.1 A systematic review combining data from CONCEPTT with that of the type 1 diabetes arm of the GlucoMOMS trial also showed evidence for a reduction in preeclampsia.
Learn More