Clinical Outcomes
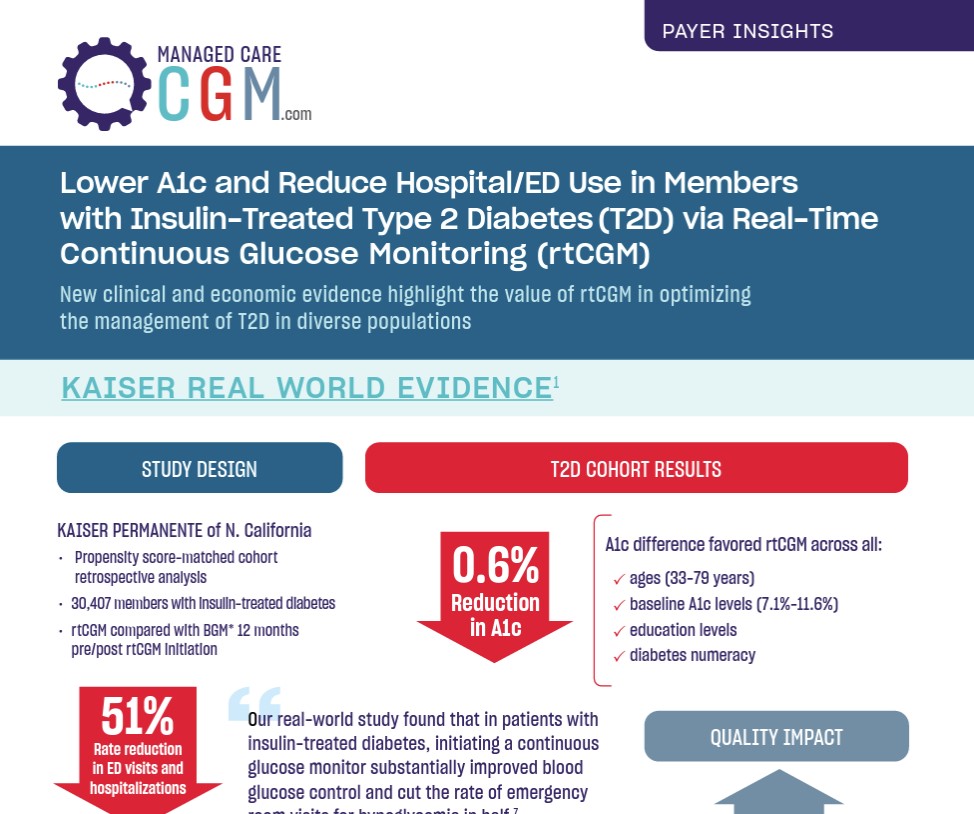
In a retrospective claims analysis from Kaiser Permanente of Northern California, 149 patients with insulin-treated type 2 diabetes (T2D; baseline HbA1c <8%) who initiated real-time continuous glucose monitoring (RT-CGM) demonstrated durable glycemic benefit compared with 17,273 noninitiators serving as reference. RT-CGM initiation was associated with decreased HbA1c (-0.06%) while noninitiation was associated with increased HbA1c (+0.32%). A weighted adjusted difference-in-difference model of change in HbA1c yielded a net benefit of -0.30% (P=0.004) for RT-CGM. These findings highlight the notion that RT-CGM may be useful in preventing glycemic deterioration and offer a durable benefit in well-controlled patients with insulin-treated T2D.
Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Continuous Glucose Monitor Use Prevents Glycemic Deterioration in Insulin-Treated Patients with Type 2 Diabetes. Diabetes Technol Ther. 2022;24(5):332-337.
Learn MoreSource: Endocrine Today
“Our real-world study found that in patients with insulin-treated diabetes, initiating a continuous glucose monitor substantially improved blood glucose control and cut the rate of emergency room visits for hypoglycemia in half,”
Andre J . Karter, PhD
Learn More