Evidence



Comparing Real-Time and Intermittently Scanned Continuous Glucose Monitoring in Adults with Type 1 Diabetes (ALERTT1): a 6-month, Prospective, Multicenter, Randomized Controlled Trial
June 9, 2021Clinical Outcomes Article / Publication
Source: The Lancet – June 2021
Key Takeaways:
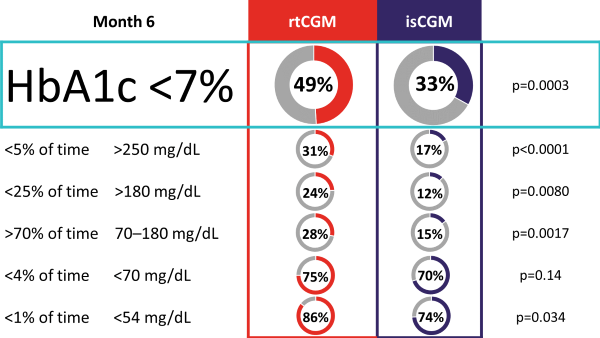
The ALERTT1 trial is the first 6-month, multicenter, prospective, randomized controlled trial comparing rtCGM with isCGM in 254 adults with type 1 diabetes, who previously used isCGM. Mean HbA1c was 7·4% (58 mmol/mol) and a minority of the study population was hypoglycemia unaware (44 [17%] people) or had a history of severe hypoglycemia (29 [11%]). Most (205 [81%]) were treated with multiple daily injections. Findings showed that in an unselected group of people with type 1 diabetes, 6-month use of rtCGM with alert functionality improved time in range (70–180 mg/dL [3.9–10.0 mmol/L]), while HbA1c, time in clinically significant hypoglycemia (< 54 mg/dL [3.0 mmol/L), and hyperglycemia (180 mg/dL [10.0 mmol/L]) were reduced. Additionally, more people on rtCGM achieved glycemic targets as defined by international consensus guidelines, and had less frequently severe hypoglycemia. Moreover, rtCGM users experienced less hypoglycemia worry and higher treatment satisfaction at the end of study.
Percentage of Participants Achieving Consensus Targets



What’s Wrong with This Picture? A Critical Review of Current Centers for Medicare & Medicaid Services Coverage Criteria for Continuous Glucose Monitoring
June 2, 2021Coverage and Benefit Design Guidelines / Policy
Source: Diabetes Technology & Therapeutics
Key Takeaway: Current CMS eligibility criteria for CGM coverage is limited and inconsistent relative to current scientific evidence. To expand access to all individuals who would benefit from CGM, it is recommended that CMS modify its eligibility requirements to include all Medicare beneficiaries who meet any one of the first four criteria below, and who also meet the fifth criterion:
Criterion Supporting Evidence 1. Diagnosed with T1D. CGM use confers:
Significant reductions in
• HbA1c
• severe hypoglycemia events
• %TBR
• diabetes-related hospitalizations Significant improvements in
• %TIR
• treatment satisfaction with less diabetes distress
2. Diagnosed with T2D and treated with any insulin regimen. CGM use confers:
Significant reductions in
• HbA1c
• %TBR
• diabetes-related hospitalizations
Significant increases in %TIR 3. Diagnosed with T2D and documented problematic hypoglycemia regardless of diabetes therapy. This would include a history of at least one of the following conditions: Level 2 (moderate) hypoglycemia, characterized by glucose levels ≤54 mg/dL; Level 3 (severe) hypoglycemia, characterized by physical/mental dysfunction requiring third-party assistance; or nocturnal hypoglycemia CGM use confers:
Significant reductions in
• diabetes-related hospitalizations, including severe hypoglycemia events
• hypoglycemia fear and Increased patient confidence in avoiding/treating hypoglycemia, thereby supporting treatment adherence
4. Advanced CKD at risk for hypoglycemia. CGM use facilitates:
• More frequent treatment changes and improved glycemic control without increased risk of hypoglycemia
• Effective monitoring and managing of glycemic levels in nondiabetes patients with ESRD undergoing dialysis 5. In-person or telemedicine consultation with the prescribing health care provider before CGM initiation and every 6 months thereafter while continuing CGM therapy. (Coverage for telemedicine consults should be available for all patients regardless of geographic location.) Use of telemedicine consults:
Significantly reduces
• the incidence of severe hypoglycemia events
• diabetes-related distress Significantly improves medication adherence
• Effectively addresses the obstacles caused by the COVID-19 pandemic
• Are more effective for patients who are residents of cities and using the websites as their
intervention method
Use of downloaded CGM data into standardized reports:
• Supports patient education
• Enhances patient engagement in their self-management
Click here to view CGM Payer Insights Sheet with key findings.
Learn More

American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus
June 2, 2021CGM Technology and Digital Health Guidelines / Policy
American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus
Source:
The American Association of Clinical Endocrinology (AACE) with a task force of medical experts developed evidence-based guideline recommendations regarding the use of advanced diabetes technology in clinical settings. The guidelines reveal that ensuring universal access to advanced diabetes technologies is anticipated to result in improved glycemia and allowing more persons with diabetes to achieve glycemic targets, improve quality of life, and potentially reduce burden of care. Furthermore, diabetes technology can improve the efficiency and effectiveness of clinical decision-making.
Featured Segments
- CGM is strongly recommended for all persons with diabetes treated with intensive insulin therapy, defined as 3 or more injections of insulin per day or an insulin pump1
- CGM is recommended for:
- All individuals with problematic hypoglycemia (frequent/severe hypoglycemia, nocturnal hypoglycemia, hypoglycemia unawareness).2
- Children/adolescents with T1D.2
- Pregnant women with T1D and T2D treated with intensive insulin therapy.2
- Women with gestational diabetes mellitus (GDM) on insulin therapy.3
- CGM may be recommended for:
- Women with GDM who are not on insulin therapy.3
- Individuals with T2D who are treated with less intensive insulin therapy.4
Real-time CGM should be recommended over intermittently scanned CGM for: isCGM should be considered for:
- persons with diabetes with problematic hypoglycemia (frequent/severe hypoglycemia, nocturnal hypoglycemia, hypoglycemia unawareness) who require predictive alarms/alerts; however the lifestyle of persons with diabetes and other factors should also be considered5
- persons with diabetes who meet 1 or more of the following criteria6
- Newly diagnosed with T2D
- Treated with nonhypoglycemic therapies
- Motivated to scan device several times per day
- At low risk for hypoglycemia, but desire more data than SMBG provides
1Grade A; High Strength of Evidence; BEL 1; 2Grade A; Intermediate-High Strength of Evidence; BEL 1; 3Grade A; Intermediate Strength of Evidence; BEL 1; 4Grade B; Intermediate Strength of Evidence, BEL 1; 5Grade B; Low-Intermediate Strength of evidence; BEL; 6Grade D; Low Strength of Evidence/Expert Opinion of Task Force; BEL
Learn More

Improving Quality Metrics and Reducing Cost of Care with Access to Real-Time Continuous Glucose Monitoring
May 18, 2021Coverage and Benefit Design Economic Outcomes Conference Updates
Source: Improving Quality Metrics and Reducing Cost of Care with Access to Real-Time Continuous Glucose Monitoring, a symposium at the Academy of Managed Care Pharmacy 2021 Virtual Annual Meeting.
Featuring expert faculty:
 Jeffrey Dunn, PharmD, MBA
Jeffrey Dunn, PharmD, MBA
Head of Clinical Pharmacy
Berkshire Hathaway/Geico
(Formerly) Vice President, Clinical Strategy and Programs and Industry Relations
Magellan Rx Management
Maria Lopes, MD, MS
Former Chief Medical Officer
Magellan Health
Former Practicing Obstetrician and Gynecologist
 Janet B. McGill, MD, MA, FACE, FACP
Janet B. McGill, MD, MA, FACE, FACP
Professor of Medicine
Washington University School of Medicine Vanita Pindolia, PharmD, BCPS, MBA
Vanita Pindolia, PharmD, BCPS, MBA
Vice President, Ambulatory Clinical Pharmacy Programs_PCM
Henry Ford Health System/Health Alliance Plan of Michigan
Key Takeaways:
- All insulin treated members, particularly high-risk older adults, should have streamlined access to real-time CGM, and payers should reconsider coverage criteria, such as removing intensive insulin eligibility criteria for T2D and streamlining the documentation requirements.
- Pharmacy coverage and access for appropriate subpopulations can confer immediate cost savings.
- Consensus guidelines recommend the use of rtCGM in pregnant women with pre-existing T1 and T2D and GDM. A delay in access to CGM can have adverse consequences in terms of both maternal and neonatal outcomes.
- rtCGM allows for a new frontier of diabetes management through remote monitoring and innovative patient engagement in telemedicine initiatives.
Jointly provided by Impact Education, LLC, and Medical Education Resources.
This activity is supported by an independent educational grant from Dexcom, Inc.
Learn MoreMay 10, 2021Clinical Outcomes Article / Publication
Source: Diabetes Technology and Therapeutics
Key Takeaway: The CONCEPTT (CGM in pregnant women with type 1 diabetes) trial provided high-quality, randomized-controlled trial data demonstrating that the use of real-time CGM was associated with lower HbA1c at 34 weeks, suggesting improved maternal glucose levels during the late second and early third trimesters. Importantly, this was accompanied by 7% higher time in range (TIR) and 5% lower time above range (TAR) without increasing maternal hypoglycemia. Beyond impacting surrogate markers of maternal glycemia, using CGM led to clinically significant reductions in large for gestational-age infants, neonatal hypoglycemia, and neonatal intensive care unit (NICU) admissions.1 A systematic review combining data from CONCEPTT with that of the type 1 diabetes arm of the GlucoMOMS trial also showed evidence for a reduction in preeclampsia.
Learn More

The Pharmacist’s Evolving Role in Diabetes Management: The Power of Real-Time Continuous Glucose Monitoring
March 29, 2021CGM Technology and Digital Health Webinar / Archive
Watch the APhA 2021 Annual Meeting and Exposition Presentation Theatre on the power of real-time continuous glucose monitoring, featuring:
Dr. Diana Isaacs
Endocrine Clinical Pharmacist & Remote Monitoring Program Coordinator
Cleveland Clinic
Dr. Jessica Haskins
Community Walgreens Site Manager
Austin, TX


Change in HbA1c and Quality of Life with Real-time CGM Use by People with Insulin-Treated Diabetes in the Landmark Study
March 1, 2021Clinical Outcomes Article / Publication
Source: Diabetes Technology and Therapeutics
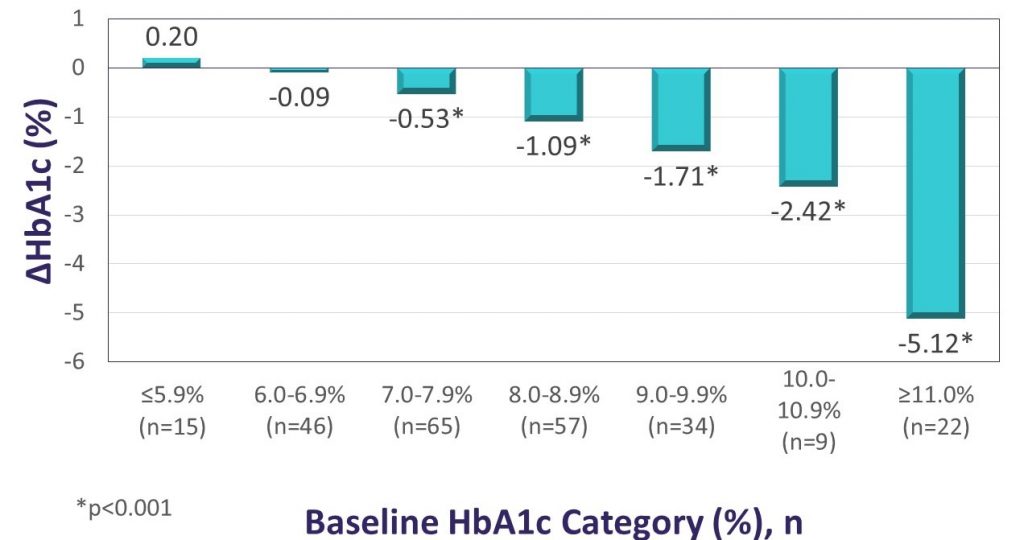
Key Takeaway: The Landmark study demonstrated significant glycemic and QoL benefits for first time CGM use among individuals using intensive insulin therapy to manage either T1D or T2D. After approximately 12 weeks of Dexcom G6 use, participants had a mean absolute reduction in HbA1c levels of 1.1%, and more than half of those with initial HbA1c values >7% experienced absolute HbA1c reductions of >1%. The reduction in HbA1c observed in Landmark was similar for patients with T1D and T2D and was more pronounced for participants with higher baseline HbA1c, consistent with observations from the DIAMOND randomized controlled trial. Significant reductions in diabetes distress and hypoglycemic concerns were also observed. In the Landmark study, there was no standardized training or intervention at CGM initiation, suggesting that the glycemic benefits can be realized without formal instruction.
Changes in HbA1c according to baseline HbA1c level
Learn MoreFebruary 26, 2021CGM Technology and Digital Health Article / Publication

Source: Association of Diabetes Care & Education Specialists and American Pharmacists Association
Key Takeaway: Developed by the Association for Diabetes Care and Education Specialists in partnership with APhA, this newly created Personal Continuous Glucose Monitoring (CGM) Implementation Playbook will help you implement a personal CGM program within your pharmacy practice.
This guide brings together fragmented information available from multiple sources to provide an inclusive and unbiased approach to implementation of Personal CGM into your practice, whatever its size. It includes a step-by-step approach to implementation, additional resources, and the latest research.
Download this free guide and start the process of incorporating this potentially game-changing tool for your patients living with diabetes.



February 26, 2021CGM Technology and Digital Health Article / PublicationSource: Association of Diabetes Care & Education Specialists and American Association of Nurse Practitioners
Key Takeaway: This toolkit provided by ADCES and AANP will help you implement a professional CGM program within your health system. Implementing a program within a healthcare setting offers many advantages, including: promotion of self-motivated, data-driven behavior change and improved clinical outcomes through alignment of medication with behavior change, resulting in lowered long-term healthcare costs for people with type 1 and type 2 diabetes.


LEARN MOREFebruary 10, 2021CGM Technology and Digital Health Article / Publication
Source: Diabetes Technology & Therapeutics
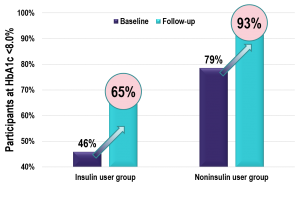
Key Takeaway: The role of real-time continuous glucose monitoring (rtCGM) is an essential component of telemedicine visits for people with diabetes. This observational study demonstrated that people with type 2 diabetes (T2D) participating in a virtual diabetes clinic can successfully insert and use Dexcom rtCGM without in-office training. The use of rtCGM was associated with a significant improvement in HbA1c at 10 months in those not meeting the ADA treatment target, independent of insulin use. In addition, there was a large shift in the percentage of participants meeting the HEDIS HbA1c target of <8.0% at follow-up; this may have important clinical and economic implications.
Chart: Percentage of Participants Achieving HEDIS HbA1c Treatment Target (HbA1c <8.0%) Before and After rtCGM Use

Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources



Comparing Real-Time and Intermittently Scanned Continuous Glucose Monitoring in Adults with Type 1 Diabetes (ALERTT1): a 6-month, Prospective, Multicenter, Randomized Controlled Trial
Source: The Lancet – June 2021
Key Takeaways:
The ALERTT1 trial is the first 6-month, multicenter, prospective, randomized controlled trial comparing rtCGM with isCGM in 254 adults with type 1 diabetes, who previously used isCGM. Mean HbA1c was 7·4% (58 mmol/mol) and a minority of the study population was hypoglycemia unaware (44 [17%] people) or had a history of severe hypoglycemia (29 [11%]). Most (205 [81%]) were treated with multiple daily injections. Findings showed that in an unselected group of people with type 1 diabetes, 6-month use of rtCGM with alert functionality improved time in range (70–180 mg/dL [3.9–10.0 mmol/L]), while HbA1c, time in clinically significant hypoglycemia (< 54 mg/dL [3.0 mmol/L), and hyperglycemia (180 mg/dL [10.0 mmol/L]) were reduced. Additionally, more people on rtCGM achieved glycemic targets as defined by international consensus guidelines, and had less frequently severe hypoglycemia. Moreover, rtCGM users experienced less hypoglycemia worry and higher treatment satisfaction at the end of study.
Percentage of Participants Achieving Consensus Targets


What’s Wrong with This Picture? A Critical Review of Current Centers for Medicare & Medicaid Services Coverage Criteria for Continuous Glucose Monitoring
June 2, 2021Coverage and Benefit Design Guidelines / Policy
Source: Diabetes Technology & Therapeutics
Key Takeaway: Current CMS eligibility criteria for CGM coverage is limited and inconsistent relative to current scientific evidence. To expand access to all individuals who would benefit from CGM, it is recommended that CMS modify its eligibility requirements to include all Medicare beneficiaries who meet any one of the first four criteria below, and who also meet the fifth criterion:
Criterion Supporting Evidence 1. Diagnosed with T1D. CGM use confers:
Significant reductions in
• HbA1c
• severe hypoglycemia events
• %TBR
• diabetes-related hospitalizations Significant improvements in
• %TIR
• treatment satisfaction with less diabetes distress
2. Diagnosed with T2D and treated with any insulin regimen. CGM use confers:
Significant reductions in
• HbA1c
• %TBR
• diabetes-related hospitalizations
Significant increases in %TIR 3. Diagnosed with T2D and documented problematic hypoglycemia regardless of diabetes therapy. This would include a history of at least one of the following conditions: Level 2 (moderate) hypoglycemia, characterized by glucose levels ≤54 mg/dL; Level 3 (severe) hypoglycemia, characterized by physical/mental dysfunction requiring third-party assistance; or nocturnal hypoglycemia CGM use confers:
Significant reductions in
• diabetes-related hospitalizations, including severe hypoglycemia events
• hypoglycemia fear and Increased patient confidence in avoiding/treating hypoglycemia, thereby supporting treatment adherence
4. Advanced CKD at risk for hypoglycemia. CGM use facilitates:
• More frequent treatment changes and improved glycemic control without increased risk of hypoglycemia
• Effective monitoring and managing of glycemic levels in nondiabetes patients with ESRD undergoing dialysis 5. In-person or telemedicine consultation with the prescribing health care provider before CGM initiation and every 6 months thereafter while continuing CGM therapy. (Coverage for telemedicine consults should be available for all patients regardless of geographic location.) Use of telemedicine consults:
Significantly reduces
• the incidence of severe hypoglycemia events
• diabetes-related distress Significantly improves medication adherence
• Effectively addresses the obstacles caused by the COVID-19 pandemic
• Are more effective for patients who are residents of cities and using the websites as their
intervention method
Use of downloaded CGM data into standardized reports:
• Supports patient education
• Enhances patient engagement in their self-management
Click here to view CGM Payer Insights Sheet with key findings.
Learn More

American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus
June 2, 2021CGM Technology and Digital Health Guidelines / Policy
American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus
Source:
The American Association of Clinical Endocrinology (AACE) with a task force of medical experts developed evidence-based guideline recommendations regarding the use of advanced diabetes technology in clinical settings. The guidelines reveal that ensuring universal access to advanced diabetes technologies is anticipated to result in improved glycemia and allowing more persons with diabetes to achieve glycemic targets, improve quality of life, and potentially reduce burden of care. Furthermore, diabetes technology can improve the efficiency and effectiveness of clinical decision-making.
Featured Segments
- CGM is strongly recommended for all persons with diabetes treated with intensive insulin therapy, defined as 3 or more injections of insulin per day or an insulin pump1
- CGM is recommended for:
- All individuals with problematic hypoglycemia (frequent/severe hypoglycemia, nocturnal hypoglycemia, hypoglycemia unawareness).2
- Children/adolescents with T1D.2
- Pregnant women with T1D and T2D treated with intensive insulin therapy.2
- Women with gestational diabetes mellitus (GDM) on insulin therapy.3
- CGM may be recommended for:
- Women with GDM who are not on insulin therapy.3
- Individuals with T2D who are treated with less intensive insulin therapy.4
Real-time CGM should be recommended over intermittently scanned CGM for: isCGM should be considered for:
- persons with diabetes with problematic hypoglycemia (frequent/severe hypoglycemia, nocturnal hypoglycemia, hypoglycemia unawareness) who require predictive alarms/alerts; however the lifestyle of persons with diabetes and other factors should also be considered5
- persons with diabetes who meet 1 or more of the following criteria6
- Newly diagnosed with T2D
- Treated with nonhypoglycemic therapies
- Motivated to scan device several times per day
- At low risk for hypoglycemia, but desire more data than SMBG provides
1Grade A; High Strength of Evidence; BEL 1; 2Grade A; Intermediate-High Strength of Evidence; BEL 1; 3Grade A; Intermediate Strength of Evidence; BEL 1; 4Grade B; Intermediate Strength of Evidence, BEL 1; 5Grade B; Low-Intermediate Strength of evidence; BEL; 6Grade D; Low Strength of Evidence/Expert Opinion of Task Force; BEL
Learn More

Improving Quality Metrics and Reducing Cost of Care with Access to Real-Time Continuous Glucose Monitoring
May 18, 2021Coverage and Benefit Design Economic Outcomes Conference Updates
Source: Improving Quality Metrics and Reducing Cost of Care with Access to Real-Time Continuous Glucose Monitoring, a symposium at the Academy of Managed Care Pharmacy 2021 Virtual Annual Meeting.
Featuring expert faculty:
 Jeffrey Dunn, PharmD, MBA
Jeffrey Dunn, PharmD, MBA
Head of Clinical Pharmacy
Berkshire Hathaway/Geico
(Formerly) Vice President, Clinical Strategy and Programs and Industry Relations
Magellan Rx Management
Maria Lopes, MD, MS
Former Chief Medical Officer
Magellan Health
Former Practicing Obstetrician and Gynecologist
 Janet B. McGill, MD, MA, FACE, FACP
Janet B. McGill, MD, MA, FACE, FACP
Professor of Medicine
Washington University School of Medicine Vanita Pindolia, PharmD, BCPS, MBA
Vanita Pindolia, PharmD, BCPS, MBA
Vice President, Ambulatory Clinical Pharmacy Programs_PCM
Henry Ford Health System/Health Alliance Plan of Michigan
Key Takeaways:
- All insulin treated members, particularly high-risk older adults, should have streamlined access to real-time CGM, and payers should reconsider coverage criteria, such as removing intensive insulin eligibility criteria for T2D and streamlining the documentation requirements.
- Pharmacy coverage and access for appropriate subpopulations can confer immediate cost savings.
- Consensus guidelines recommend the use of rtCGM in pregnant women with pre-existing T1 and T2D and GDM. A delay in access to CGM can have adverse consequences in terms of both maternal and neonatal outcomes.
- rtCGM allows for a new frontier of diabetes management through remote monitoring and innovative patient engagement in telemedicine initiatives.
Jointly provided by Impact Education, LLC, and Medical Education Resources.
This activity is supported by an independent educational grant from Dexcom, Inc.
Learn MoreMay 10, 2021Clinical Outcomes Article / Publication
Source: Diabetes Technology and Therapeutics
Key Takeaway: The CONCEPTT (CGM in pregnant women with type 1 diabetes) trial provided high-quality, randomized-controlled trial data demonstrating that the use of real-time CGM was associated with lower HbA1c at 34 weeks, suggesting improved maternal glucose levels during the late second and early third trimesters. Importantly, this was accompanied by 7% higher time in range (TIR) and 5% lower time above range (TAR) without increasing maternal hypoglycemia. Beyond impacting surrogate markers of maternal glycemia, using CGM led to clinically significant reductions in large for gestational-age infants, neonatal hypoglycemia, and neonatal intensive care unit (NICU) admissions.1 A systematic review combining data from CONCEPTT with that of the type 1 diabetes arm of the GlucoMOMS trial also showed evidence for a reduction in preeclampsia.
Learn More

The Pharmacist’s Evolving Role in Diabetes Management: The Power of Real-Time Continuous Glucose Monitoring
March 29, 2021CGM Technology and Digital Health Webinar / Archive
Watch the APhA 2021 Annual Meeting and Exposition Presentation Theatre on the power of real-time continuous glucose monitoring, featuring:
Dr. Diana Isaacs
Endocrine Clinical Pharmacist & Remote Monitoring Program Coordinator
Cleveland Clinic
Dr. Jessica Haskins
Community Walgreens Site Manager
Austin, TX


Change in HbA1c and Quality of Life with Real-time CGM Use by People with Insulin-Treated Diabetes in the Landmark Study
March 1, 2021Clinical Outcomes Article / Publication
Source: Diabetes Technology and Therapeutics
Key Takeaway: The Landmark study demonstrated significant glycemic and QoL benefits for first time CGM use among individuals using intensive insulin therapy to manage either T1D or T2D. After approximately 12 weeks of Dexcom G6 use, participants had a mean absolute reduction in HbA1c levels of 1.1%, and more than half of those with initial HbA1c values >7% experienced absolute HbA1c reductions of >1%. The reduction in HbA1c observed in Landmark was similar for patients with T1D and T2D and was more pronounced for participants with higher baseline HbA1c, consistent with observations from the DIAMOND randomized controlled trial. Significant reductions in diabetes distress and hypoglycemic concerns were also observed. In the Landmark study, there was no standardized training or intervention at CGM initiation, suggesting that the glycemic benefits can be realized without formal instruction.
Changes in HbA1c according to baseline HbA1c level
Learn MoreFebruary 26, 2021CGM Technology and Digital Health Article / Publication

Source: Association of Diabetes Care & Education Specialists and American Pharmacists Association
Key Takeaway: Developed by the Association for Diabetes Care and Education Specialists in partnership with APhA, this newly created Personal Continuous Glucose Monitoring (CGM) Implementation Playbook will help you implement a personal CGM program within your pharmacy practice.
This guide brings together fragmented information available from multiple sources to provide an inclusive and unbiased approach to implementation of Personal CGM into your practice, whatever its size. It includes a step-by-step approach to implementation, additional resources, and the latest research.
Download this free guide and start the process of incorporating this potentially game-changing tool for your patients living with diabetes.



February 26, 2021CGM Technology and Digital Health Article / PublicationSource: Association of Diabetes Care & Education Specialists and American Association of Nurse Practitioners
Key Takeaway: This toolkit provided by ADCES and AANP will help you implement a professional CGM program within your health system. Implementing a program within a healthcare setting offers many advantages, including: promotion of self-motivated, data-driven behavior change and improved clinical outcomes through alignment of medication with behavior change, resulting in lowered long-term healthcare costs for people with type 1 and type 2 diabetes.


LEARN MOREFebruary 10, 2021CGM Technology and Digital Health Article / Publication
Source: Diabetes Technology & Therapeutics
Key Takeaway: The role of real-time continuous glucose monitoring (rtCGM) is an essential component of telemedicine visits for people with diabetes. This observational study demonstrated that people with type 2 diabetes (T2D) participating in a virtual diabetes clinic can successfully insert and use Dexcom rtCGM without in-office training. The use of rtCGM was associated with a significant improvement in HbA1c at 10 months in those not meeting the ADA treatment target, independent of insulin use. In addition, there was a large shift in the percentage of participants meeting the HEDIS HbA1c target of <8.0% at follow-up; this may have important clinical and economic implications.
Chart: Percentage of Participants Achieving HEDIS HbA1c Treatment Target (HbA1c <8.0%) Before and After rtCGM Use

Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources


What’s Wrong with This Picture? A Critical Review of Current Centers for Medicare & Medicaid Services Coverage Criteria for Continuous Glucose Monitoring
Source: Diabetes Technology & Therapeutics
Key Takeaway: Current CMS eligibility criteria for CGM coverage is limited and inconsistent relative to current scientific evidence. To expand access to all individuals who would benefit from CGM, it is recommended that CMS modify its eligibility requirements to include all Medicare beneficiaries who meet any one of the first four criteria below, and who also meet the fifth criterion:
| Criterion | Supporting Evidence |
| 1. Diagnosed with T1D. | CGM use confers: Significant reductions in • HbA1c • severe hypoglycemia events • %TBR • diabetes-related hospitalizations Significant improvements in |
| 2. Diagnosed with T2D and treated with any insulin regimen. | CGM use confers: Significant reductions in • HbA1c • %TBR • diabetes-related hospitalizations Significant increases in %TIR |
| 3. Diagnosed with T2D and documented problematic hypoglycemia regardless of diabetes therapy. This would include a history of at least one of the following conditions: Level 2 (moderate) hypoglycemia, characterized by glucose levels ≤54 mg/dL; Level 3 (severe) hypoglycemia, characterized by physical/mental dysfunction requiring third-party assistance; or nocturnal hypoglycemia | CGM use confers: Significant reductions in • diabetes-related hospitalizations, including severe hypoglycemia events • hypoglycemia fear and Increased patient confidence in avoiding/treating hypoglycemia, thereby supporting treatment adherence |
| 4. Advanced CKD at risk for hypoglycemia. | CGM use facilitates: • More frequent treatment changes and improved glycemic control without increased risk of hypoglycemia • Effective monitoring and managing of glycemic levels in nondiabetes patients with ESRD undergoing dialysis |
| 5. In-person or telemedicine consultation with the prescribing health care provider before CGM initiation and every 6 months thereafter while continuing CGM therapy. (Coverage for telemedicine consults should be available for all patients regardless of geographic location.) | Use of telemedicine consults: Significantly reduces • the incidence of severe hypoglycemia events • diabetes-related distress Significantly improves medication adherence Use of downloaded CGM data into standardized reports: |
Click here to view CGM Payer Insights Sheet with key findings.
Learn More

American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus
June 2, 2021CGM Technology and Digital Health Guidelines / Policy
American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus
Source:
The American Association of Clinical Endocrinology (AACE) with a task force of medical experts developed evidence-based guideline recommendations regarding the use of advanced diabetes technology in clinical settings. The guidelines reveal that ensuring universal access to advanced diabetes technologies is anticipated to result in improved glycemia and allowing more persons with diabetes to achieve glycemic targets, improve quality of life, and potentially reduce burden of care. Furthermore, diabetes technology can improve the efficiency and effectiveness of clinical decision-making.
Featured Segments
- CGM is strongly recommended for all persons with diabetes treated with intensive insulin therapy, defined as 3 or more injections of insulin per day or an insulin pump1
- CGM is recommended for:
- All individuals with problematic hypoglycemia (frequent/severe hypoglycemia, nocturnal hypoglycemia, hypoglycemia unawareness).2
- Children/adolescents with T1D.2
- Pregnant women with T1D and T2D treated with intensive insulin therapy.2
- Women with gestational diabetes mellitus (GDM) on insulin therapy.3
- CGM may be recommended for:
- Women with GDM who are not on insulin therapy.3
- Individuals with T2D who are treated with less intensive insulin therapy.4
Real-time CGM should be recommended over intermittently scanned CGM for: isCGM should be considered for:
- persons with diabetes with problematic hypoglycemia (frequent/severe hypoglycemia, nocturnal hypoglycemia, hypoglycemia unawareness) who require predictive alarms/alerts; however the lifestyle of persons with diabetes and other factors should also be considered5
- persons with diabetes who meet 1 or more of the following criteria6
- Newly diagnosed with T2D
- Treated with nonhypoglycemic therapies
- Motivated to scan device several times per day
- At low risk for hypoglycemia, but desire more data than SMBG provides
1Grade A; High Strength of Evidence; BEL 1; 2Grade A; Intermediate-High Strength of Evidence; BEL 1; 3Grade A; Intermediate Strength of Evidence; BEL 1; 4Grade B; Intermediate Strength of Evidence, BEL 1; 5Grade B; Low-Intermediate Strength of evidence; BEL; 6Grade D; Low Strength of Evidence/Expert Opinion of Task Force; BEL
Learn More

Improving Quality Metrics and Reducing Cost of Care with Access to Real-Time Continuous Glucose Monitoring
May 18, 2021Coverage and Benefit Design Economic Outcomes Conference Updates
Source: Improving Quality Metrics and Reducing Cost of Care with Access to Real-Time Continuous Glucose Monitoring, a symposium at the Academy of Managed Care Pharmacy 2021 Virtual Annual Meeting.
Featuring expert faculty:
 Jeffrey Dunn, PharmD, MBA
Jeffrey Dunn, PharmD, MBA
Head of Clinical Pharmacy
Berkshire Hathaway/Geico
(Formerly) Vice President, Clinical Strategy and Programs and Industry Relations
Magellan Rx Management
Maria Lopes, MD, MS
Former Chief Medical Officer
Magellan Health
Former Practicing Obstetrician and Gynecologist
 Janet B. McGill, MD, MA, FACE, FACP
Janet B. McGill, MD, MA, FACE, FACP
Professor of Medicine
Washington University School of Medicine Vanita Pindolia, PharmD, BCPS, MBA
Vanita Pindolia, PharmD, BCPS, MBA
Vice President, Ambulatory Clinical Pharmacy Programs_PCM
Henry Ford Health System/Health Alliance Plan of Michigan
Key Takeaways:
- All insulin treated members, particularly high-risk older adults, should have streamlined access to real-time CGM, and payers should reconsider coverage criteria, such as removing intensive insulin eligibility criteria for T2D and streamlining the documentation requirements.
- Pharmacy coverage and access for appropriate subpopulations can confer immediate cost savings.
- Consensus guidelines recommend the use of rtCGM in pregnant women with pre-existing T1 and T2D and GDM. A delay in access to CGM can have adverse consequences in terms of both maternal and neonatal outcomes.
- rtCGM allows for a new frontier of diabetes management through remote monitoring and innovative patient engagement in telemedicine initiatives.
Jointly provided by Impact Education, LLC, and Medical Education Resources.
This activity is supported by an independent educational grant from Dexcom, Inc.
Learn MoreMay 10, 2021Clinical Outcomes Article / Publication
Source: Diabetes Technology and Therapeutics
Key Takeaway: The CONCEPTT (CGM in pregnant women with type 1 diabetes) trial provided high-quality, randomized-controlled trial data demonstrating that the use of real-time CGM was associated with lower HbA1c at 34 weeks, suggesting improved maternal glucose levels during the late second and early third trimesters. Importantly, this was accompanied by 7% higher time in range (TIR) and 5% lower time above range (TAR) without increasing maternal hypoglycemia. Beyond impacting surrogate markers of maternal glycemia, using CGM led to clinically significant reductions in large for gestational-age infants, neonatal hypoglycemia, and neonatal intensive care unit (NICU) admissions.1 A systematic review combining data from CONCEPTT with that of the type 1 diabetes arm of the GlucoMOMS trial also showed evidence for a reduction in preeclampsia.
Learn More

The Pharmacist’s Evolving Role in Diabetes Management: The Power of Real-Time Continuous Glucose Monitoring
March 29, 2021CGM Technology and Digital Health Webinar / Archive
Watch the APhA 2021 Annual Meeting and Exposition Presentation Theatre on the power of real-time continuous glucose monitoring, featuring:
Dr. Diana Isaacs
Endocrine Clinical Pharmacist & Remote Monitoring Program Coordinator
Cleveland Clinic
Dr. Jessica Haskins
Community Walgreens Site Manager
Austin, TX


Change in HbA1c and Quality of Life with Real-time CGM Use by People with Insulin-Treated Diabetes in the Landmark Study
March 1, 2021Clinical Outcomes Article / Publication
Source: Diabetes Technology and Therapeutics
Key Takeaway: The Landmark study demonstrated significant glycemic and QoL benefits for first time CGM use among individuals using intensive insulin therapy to manage either T1D or T2D. After approximately 12 weeks of Dexcom G6 use, participants had a mean absolute reduction in HbA1c levels of 1.1%, and more than half of those with initial HbA1c values >7% experienced absolute HbA1c reductions of >1%. The reduction in HbA1c observed in Landmark was similar for patients with T1D and T2D and was more pronounced for participants with higher baseline HbA1c, consistent with observations from the DIAMOND randomized controlled trial. Significant reductions in diabetes distress and hypoglycemic concerns were also observed. In the Landmark study, there was no standardized training or intervention at CGM initiation, suggesting that the glycemic benefits can be realized without formal instruction.
Changes in HbA1c according to baseline HbA1c level
Learn MoreFebruary 26, 2021CGM Technology and Digital Health Article / Publication

Source: Association of Diabetes Care & Education Specialists and American Pharmacists Association
Key Takeaway: Developed by the Association for Diabetes Care and Education Specialists in partnership with APhA, this newly created Personal Continuous Glucose Monitoring (CGM) Implementation Playbook will help you implement a personal CGM program within your pharmacy practice.
This guide brings together fragmented information available from multiple sources to provide an inclusive and unbiased approach to implementation of Personal CGM into your practice, whatever its size. It includes a step-by-step approach to implementation, additional resources, and the latest research.
Download this free guide and start the process of incorporating this potentially game-changing tool for your patients living with diabetes.



February 26, 2021CGM Technology and Digital Health Article / PublicationSource: Association of Diabetes Care & Education Specialists and American Association of Nurse Practitioners
Key Takeaway: This toolkit provided by ADCES and AANP will help you implement a professional CGM program within your health system. Implementing a program within a healthcare setting offers many advantages, including: promotion of self-motivated, data-driven behavior change and improved clinical outcomes through alignment of medication with behavior change, resulting in lowered long-term healthcare costs for people with type 1 and type 2 diabetes.


LEARN MOREFebruary 10, 2021CGM Technology and Digital Health Article / Publication
Source: Diabetes Technology & Therapeutics
Key Takeaway: The role of real-time continuous glucose monitoring (rtCGM) is an essential component of telemedicine visits for people with diabetes. This observational study demonstrated that people with type 2 diabetes (T2D) participating in a virtual diabetes clinic can successfully insert and use Dexcom rtCGM without in-office training. The use of rtCGM was associated with a significant improvement in HbA1c at 10 months in those not meeting the ADA treatment target, independent of insulin use. In addition, there was a large shift in the percentage of participants meeting the HEDIS HbA1c target of <8.0% at follow-up; this may have important clinical and economic implications.
Chart: Percentage of Participants Achieving HEDIS HbA1c Treatment Target (HbA1c <8.0%) Before and After rtCGM Use

Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources


American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus
American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus
Source:
The American Association of Clinical Endocrinology (AACE) with a task force of medical experts developed evidence-based guideline recommendations regarding the use of advanced diabetes technology in clinical settings. The guidelines reveal that ensuring universal access to advanced diabetes technologies is anticipated to result in improved glycemia and allowing more persons with diabetes to achieve glycemic targets, improve quality of life, and potentially reduce burden of care. Furthermore, diabetes technology can improve the efficiency and effectiveness of clinical decision-making.
Featured Segments
- CGM is strongly recommended for all persons with diabetes treated with intensive insulin therapy, defined as 3 or more injections of insulin per day or an insulin pump1
- CGM is recommended for:
- All individuals with problematic hypoglycemia (frequent/severe hypoglycemia, nocturnal hypoglycemia, hypoglycemia unawareness).2
- Children/adolescents with T1D.2
- Pregnant women with T1D and T2D treated with intensive insulin therapy.2
- Women with gestational diabetes mellitus (GDM) on insulin therapy.3
- CGM may be recommended for:
- Women with GDM who are not on insulin therapy.3
- Individuals with T2D who are treated with less intensive insulin therapy.4
| Real-time CGM should be recommended over intermittently scanned CGM for: | isCGM should be considered for: |
|
|
1Grade A; High Strength of Evidence; BEL 1; 2Grade A; Intermediate-High Strength of Evidence; BEL 1; 3Grade A; Intermediate Strength of Evidence; BEL 1; 4Grade B; Intermediate Strength of Evidence, BEL 1; 5Grade B; Low-Intermediate Strength of evidence; BEL; 6Grade D; Low Strength of Evidence/Expert Opinion of Task Force; BEL
Learn More

Improving Quality Metrics and Reducing Cost of Care with Access to Real-Time Continuous Glucose Monitoring
May 18, 2021Coverage and Benefit Design Economic Outcomes Conference Updates
Source: Improving Quality Metrics and Reducing Cost of Care with Access to Real-Time Continuous Glucose Monitoring, a symposium at the Academy of Managed Care Pharmacy 2021 Virtual Annual Meeting.
Featuring expert faculty:
 Jeffrey Dunn, PharmD, MBA
Jeffrey Dunn, PharmD, MBA
Head of Clinical Pharmacy
Berkshire Hathaway/Geico
(Formerly) Vice President, Clinical Strategy and Programs and Industry Relations
Magellan Rx Management
Maria Lopes, MD, MS
Former Chief Medical Officer
Magellan Health
Former Practicing Obstetrician and Gynecologist
 Janet B. McGill, MD, MA, FACE, FACP
Janet B. McGill, MD, MA, FACE, FACP
Professor of Medicine
Washington University School of Medicine Vanita Pindolia, PharmD, BCPS, MBA
Vanita Pindolia, PharmD, BCPS, MBA
Vice President, Ambulatory Clinical Pharmacy Programs_PCM
Henry Ford Health System/Health Alliance Plan of Michigan
Key Takeaways:
- All insulin treated members, particularly high-risk older adults, should have streamlined access to real-time CGM, and payers should reconsider coverage criteria, such as removing intensive insulin eligibility criteria for T2D and streamlining the documentation requirements.
- Pharmacy coverage and access for appropriate subpopulations can confer immediate cost savings.
- Consensus guidelines recommend the use of rtCGM in pregnant women with pre-existing T1 and T2D and GDM. A delay in access to CGM can have adverse consequences in terms of both maternal and neonatal outcomes.
- rtCGM allows for a new frontier of diabetes management through remote monitoring and innovative patient engagement in telemedicine initiatives.
Jointly provided by Impact Education, LLC, and Medical Education Resources.
This activity is supported by an independent educational grant from Dexcom, Inc.
Learn MoreMay 10, 2021Clinical Outcomes Article / Publication
Source: Diabetes Technology and Therapeutics
Key Takeaway: The CONCEPTT (CGM in pregnant women with type 1 diabetes) trial provided high-quality, randomized-controlled trial data demonstrating that the use of real-time CGM was associated with lower HbA1c at 34 weeks, suggesting improved maternal glucose levels during the late second and early third trimesters. Importantly, this was accompanied by 7% higher time in range (TIR) and 5% lower time above range (TAR) without increasing maternal hypoglycemia. Beyond impacting surrogate markers of maternal glycemia, using CGM led to clinically significant reductions in large for gestational-age infants, neonatal hypoglycemia, and neonatal intensive care unit (NICU) admissions.1 A systematic review combining data from CONCEPTT with that of the type 1 diabetes arm of the GlucoMOMS trial also showed evidence for a reduction in preeclampsia.
Learn More

The Pharmacist’s Evolving Role in Diabetes Management: The Power of Real-Time Continuous Glucose Monitoring
March 29, 2021CGM Technology and Digital Health Webinar / Archive
Watch the APhA 2021 Annual Meeting and Exposition Presentation Theatre on the power of real-time continuous glucose monitoring, featuring:
Dr. Diana Isaacs
Endocrine Clinical Pharmacist & Remote Monitoring Program Coordinator
Cleveland Clinic
Dr. Jessica Haskins
Community Walgreens Site Manager
Austin, TX


Change in HbA1c and Quality of Life with Real-time CGM Use by People with Insulin-Treated Diabetes in the Landmark Study
March 1, 2021Clinical Outcomes Article / Publication
Source: Diabetes Technology and Therapeutics
Key Takeaway: The Landmark study demonstrated significant glycemic and QoL benefits for first time CGM use among individuals using intensive insulin therapy to manage either T1D or T2D. After approximately 12 weeks of Dexcom G6 use, participants had a mean absolute reduction in HbA1c levels of 1.1%, and more than half of those with initial HbA1c values >7% experienced absolute HbA1c reductions of >1%. The reduction in HbA1c observed in Landmark was similar for patients with T1D and T2D and was more pronounced for participants with higher baseline HbA1c, consistent with observations from the DIAMOND randomized controlled trial. Significant reductions in diabetes distress and hypoglycemic concerns were also observed. In the Landmark study, there was no standardized training or intervention at CGM initiation, suggesting that the glycemic benefits can be realized without formal instruction.
Changes in HbA1c according to baseline HbA1c level
Learn MoreFebruary 26, 2021CGM Technology and Digital Health Article / Publication

Source: Association of Diabetes Care & Education Specialists and American Pharmacists Association
Key Takeaway: Developed by the Association for Diabetes Care and Education Specialists in partnership with APhA, this newly created Personal Continuous Glucose Monitoring (CGM) Implementation Playbook will help you implement a personal CGM program within your pharmacy practice.
This guide brings together fragmented information available from multiple sources to provide an inclusive and unbiased approach to implementation of Personal CGM into your practice, whatever its size. It includes a step-by-step approach to implementation, additional resources, and the latest research.
Download this free guide and start the process of incorporating this potentially game-changing tool for your patients living with diabetes.



February 26, 2021CGM Technology and Digital Health Article / PublicationSource: Association of Diabetes Care & Education Specialists and American Association of Nurse Practitioners
Key Takeaway: This toolkit provided by ADCES and AANP will help you implement a professional CGM program within your health system. Implementing a program within a healthcare setting offers many advantages, including: promotion of self-motivated, data-driven behavior change and improved clinical outcomes through alignment of medication with behavior change, resulting in lowered long-term healthcare costs for people with type 1 and type 2 diabetes.


LEARN MOREFebruary 10, 2021CGM Technology and Digital Health Article / Publication
Source: Diabetes Technology & Therapeutics
Key Takeaway: The role of real-time continuous glucose monitoring (rtCGM) is an essential component of telemedicine visits for people with diabetes. This observational study demonstrated that people with type 2 diabetes (T2D) participating in a virtual diabetes clinic can successfully insert and use Dexcom rtCGM without in-office training. The use of rtCGM was associated with a significant improvement in HbA1c at 10 months in those not meeting the ADA treatment target, independent of insulin use. In addition, there was a large shift in the percentage of participants meeting the HEDIS HbA1c target of <8.0% at follow-up; this may have important clinical and economic implications.
Chart: Percentage of Participants Achieving HEDIS HbA1c Treatment Target (HbA1c <8.0%) Before and After rtCGM Use

Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources


Improving Quality Metrics and Reducing Cost of Care with Access to Real-Time Continuous Glucose Monitoring
Source: Improving Quality Metrics and Reducing Cost of Care with Access to Real-Time Continuous Glucose Monitoring, a symposium at the Academy of Managed Care Pharmacy 2021 Virtual Annual Meeting.
Featuring expert faculty:
 Jeffrey Dunn, PharmD, MBA Jeffrey Dunn, PharmD, MBAHead of Clinical Pharmacy Berkshire Hathaway/Geico (Formerly) Vice President, Clinical Strategy and Programs and Industry Relations Magellan Rx Management |  Maria Lopes, MD, MS |
 Janet B. McGill, MD, MA, FACE, FACP Janet B. McGill, MD, MA, FACE, FACPProfessor of Medicine Washington University School of Medicine |  Vanita Pindolia, PharmD, BCPS, MBA Vanita Pindolia, PharmD, BCPS, MBAVice President, Ambulatory Clinical Pharmacy Programs_PCM Henry Ford Health System/Health Alliance Plan of Michigan |
Key Takeaways:
- All insulin treated members, particularly high-risk older adults, should have streamlined access to real-time CGM, and payers should reconsider coverage criteria, such as removing intensive insulin eligibility criteria for T2D and streamlining the documentation requirements.
- Pharmacy coverage and access for appropriate subpopulations can confer immediate cost savings.
- Consensus guidelines recommend the use of rtCGM in pregnant women with pre-existing T1 and T2D and GDM. A delay in access to CGM can have adverse consequences in terms of both maternal and neonatal outcomes.
- rtCGM allows for a new frontier of diabetes management through remote monitoring and innovative patient engagement in telemedicine initiatives.
Jointly provided by Impact Education, LLC, and Medical Education Resources.
This activity is supported by an independent educational grant from Dexcom, Inc.
May 10, 2021Clinical Outcomes Article / Publication
Source: Diabetes Technology and Therapeutics
Key Takeaway: The CONCEPTT (CGM in pregnant women with type 1 diabetes) trial provided high-quality, randomized-controlled trial data demonstrating that the use of real-time CGM was associated with lower HbA1c at 34 weeks, suggesting improved maternal glucose levels during the late second and early third trimesters. Importantly, this was accompanied by 7% higher time in range (TIR) and 5% lower time above range (TAR) without increasing maternal hypoglycemia. Beyond impacting surrogate markers of maternal glycemia, using CGM led to clinically significant reductions in large for gestational-age infants, neonatal hypoglycemia, and neonatal intensive care unit (NICU) admissions.1 A systematic review combining data from CONCEPTT with that of the type 1 diabetes arm of the GlucoMOMS trial also showed evidence for a reduction in preeclampsia.
Learn More

The Pharmacist’s Evolving Role in Diabetes Management: The Power of Real-Time Continuous Glucose Monitoring
March 29, 2021CGM Technology and Digital Health Webinar / Archive
Watch the APhA 2021 Annual Meeting and Exposition Presentation Theatre on the power of real-time continuous glucose monitoring, featuring:
Dr. Diana Isaacs
Endocrine Clinical Pharmacist & Remote Monitoring Program Coordinator
Cleveland Clinic
Dr. Jessica Haskins
Community Walgreens Site Manager
Austin, TX


Change in HbA1c and Quality of Life with Real-time CGM Use by People with Insulin-Treated Diabetes in the Landmark Study
March 1, 2021Clinical Outcomes Article / Publication
Source: Diabetes Technology and Therapeutics
Key Takeaway: The Landmark study demonstrated significant glycemic and QoL benefits for first time CGM use among individuals using intensive insulin therapy to manage either T1D or T2D. After approximately 12 weeks of Dexcom G6 use, participants had a mean absolute reduction in HbA1c levels of 1.1%, and more than half of those with initial HbA1c values >7% experienced absolute HbA1c reductions of >1%. The reduction in HbA1c observed in Landmark was similar for patients with T1D and T2D and was more pronounced for participants with higher baseline HbA1c, consistent with observations from the DIAMOND randomized controlled trial. Significant reductions in diabetes distress and hypoglycemic concerns were also observed. In the Landmark study, there was no standardized training or intervention at CGM initiation, suggesting that the glycemic benefits can be realized without formal instruction.
Changes in HbA1c according to baseline HbA1c level
Learn MoreFebruary 26, 2021CGM Technology and Digital Health Article / Publication

Source: Association of Diabetes Care & Education Specialists and American Pharmacists Association
Key Takeaway: Developed by the Association for Diabetes Care and Education Specialists in partnership with APhA, this newly created Personal Continuous Glucose Monitoring (CGM) Implementation Playbook will help you implement a personal CGM program within your pharmacy practice.
This guide brings together fragmented information available from multiple sources to provide an inclusive and unbiased approach to implementation of Personal CGM into your practice, whatever its size. It includes a step-by-step approach to implementation, additional resources, and the latest research.
Download this free guide and start the process of incorporating this potentially game-changing tool for your patients living with diabetes.



February 26, 2021CGM Technology and Digital Health Article / PublicationSource: Association of Diabetes Care & Education Specialists and American Association of Nurse Practitioners
Key Takeaway: This toolkit provided by ADCES and AANP will help you implement a professional CGM program within your health system. Implementing a program within a healthcare setting offers many advantages, including: promotion of self-motivated, data-driven behavior change and improved clinical outcomes through alignment of medication with behavior change, resulting in lowered long-term healthcare costs for people with type 1 and type 2 diabetes.


LEARN MOREFebruary 10, 2021CGM Technology and Digital Health Article / Publication
Source: Diabetes Technology & Therapeutics
Key Takeaway: The role of real-time continuous glucose monitoring (rtCGM) is an essential component of telemedicine visits for people with diabetes. This observational study demonstrated that people with type 2 diabetes (T2D) participating in a virtual diabetes clinic can successfully insert and use Dexcom rtCGM without in-office training. The use of rtCGM was associated with a significant improvement in HbA1c at 10 months in those not meeting the ADA treatment target, independent of insulin use. In addition, there was a large shift in the percentage of participants meeting the HEDIS HbA1c target of <8.0% at follow-up; this may have important clinical and economic implications.
Chart: Percentage of Participants Achieving HEDIS HbA1c Treatment Target (HbA1c <8.0%) Before and After rtCGM Use

Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources
Source: Diabetes Technology and Therapeutics
Key Takeaway: The CONCEPTT (CGM in pregnant women with type 1 diabetes) trial provided high-quality, randomized-controlled trial data demonstrating that the use of real-time CGM was associated with lower HbA1c at 34 weeks, suggesting improved maternal glucose levels during the late second and early third trimesters. Importantly, this was accompanied by 7% higher time in range (TIR) and 5% lower time above range (TAR) without increasing maternal hypoglycemia. Beyond impacting surrogate markers of maternal glycemia, using CGM led to clinically significant reductions in large for gestational-age infants, neonatal hypoglycemia, and neonatal intensive care unit (NICU) admissions.1 A systematic review combining data from CONCEPTT with that of the type 1 diabetes arm of the GlucoMOMS trial also showed evidence for a reduction in preeclampsia.
Learn More

The Pharmacist’s Evolving Role in Diabetes Management: The Power of Real-Time Continuous Glucose Monitoring
March 29, 2021CGM Technology and Digital Health Webinar / Archive
Watch the APhA 2021 Annual Meeting and Exposition Presentation Theatre on the power of real-time continuous glucose monitoring, featuring:
Dr. Diana Isaacs
Endocrine Clinical Pharmacist & Remote Monitoring Program Coordinator
Cleveland Clinic
Dr. Jessica Haskins
Community Walgreens Site Manager
Austin, TX


Change in HbA1c and Quality of Life with Real-time CGM Use by People with Insulin-Treated Diabetes in the Landmark Study
March 1, 2021Clinical Outcomes Article / Publication
Source: Diabetes Technology and Therapeutics
Key Takeaway: The Landmark study demonstrated significant glycemic and QoL benefits for first time CGM use among individuals using intensive insulin therapy to manage either T1D or T2D. After approximately 12 weeks of Dexcom G6 use, participants had a mean absolute reduction in HbA1c levels of 1.1%, and more than half of those with initial HbA1c values >7% experienced absolute HbA1c reductions of >1%. The reduction in HbA1c observed in Landmark was similar for patients with T1D and T2D and was more pronounced for participants with higher baseline HbA1c, consistent with observations from the DIAMOND randomized controlled trial. Significant reductions in diabetes distress and hypoglycemic concerns were also observed. In the Landmark study, there was no standardized training or intervention at CGM initiation, suggesting that the glycemic benefits can be realized without formal instruction.
Changes in HbA1c according to baseline HbA1c level
Learn MoreFebruary 26, 2021CGM Technology and Digital Health Article / Publication

Source: Association of Diabetes Care & Education Specialists and American Pharmacists Association
Key Takeaway: Developed by the Association for Diabetes Care and Education Specialists in partnership with APhA, this newly created Personal Continuous Glucose Monitoring (CGM) Implementation Playbook will help you implement a personal CGM program within your pharmacy practice.
This guide brings together fragmented information available from multiple sources to provide an inclusive and unbiased approach to implementation of Personal CGM into your practice, whatever its size. It includes a step-by-step approach to implementation, additional resources, and the latest research.
Download this free guide and start the process of incorporating this potentially game-changing tool for your patients living with diabetes.



February 26, 2021CGM Technology and Digital Health Article / PublicationSource: Association of Diabetes Care & Education Specialists and American Association of Nurse Practitioners
Key Takeaway: This toolkit provided by ADCES and AANP will help you implement a professional CGM program within your health system. Implementing a program within a healthcare setting offers many advantages, including: promotion of self-motivated, data-driven behavior change and improved clinical outcomes through alignment of medication with behavior change, resulting in lowered long-term healthcare costs for people with type 1 and type 2 diabetes.


LEARN MOREFebruary 10, 2021CGM Technology and Digital Health Article / Publication
Source: Diabetes Technology & Therapeutics
Key Takeaway: The role of real-time continuous glucose monitoring (rtCGM) is an essential component of telemedicine visits for people with diabetes. This observational study demonstrated that people with type 2 diabetes (T2D) participating in a virtual diabetes clinic can successfully insert and use Dexcom rtCGM without in-office training. The use of rtCGM was associated with a significant improvement in HbA1c at 10 months in those not meeting the ADA treatment target, independent of insulin use. In addition, there was a large shift in the percentage of participants meeting the HEDIS HbA1c target of <8.0% at follow-up; this may have important clinical and economic implications.
Chart: Percentage of Participants Achieving HEDIS HbA1c Treatment Target (HbA1c <8.0%) Before and After rtCGM Use

Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources


The Pharmacist’s Evolving Role in Diabetes Management: The Power of Real-Time Continuous Glucose Monitoring
Watch the APhA 2021 Annual Meeting and Exposition Presentation Theatre on the power of real-time continuous glucose monitoring, featuring:
Dr. Diana Isaacs
Endocrine Clinical Pharmacist & Remote Monitoring Program Coordinator
Cleveland Clinic
Dr. Jessica Haskins
Community Walgreens Site Manager
Austin, TX


Change in HbA1c and Quality of Life with Real-time CGM Use by People with Insulin-Treated Diabetes in the Landmark Study
March 1, 2021Clinical Outcomes Article / Publication
Source: Diabetes Technology and Therapeutics
Key Takeaway: The Landmark study demonstrated significant glycemic and QoL benefits for first time CGM use among individuals using intensive insulin therapy to manage either T1D or T2D. After approximately 12 weeks of Dexcom G6 use, participants had a mean absolute reduction in HbA1c levels of 1.1%, and more than half of those with initial HbA1c values >7% experienced absolute HbA1c reductions of >1%. The reduction in HbA1c observed in Landmark was similar for patients with T1D and T2D and was more pronounced for participants with higher baseline HbA1c, consistent with observations from the DIAMOND randomized controlled trial. Significant reductions in diabetes distress and hypoglycemic concerns were also observed. In the Landmark study, there was no standardized training or intervention at CGM initiation, suggesting that the glycemic benefits can be realized without formal instruction.
Changes in HbA1c according to baseline HbA1c level
Learn MoreFebruary 26, 2021CGM Technology and Digital Health Article / Publication

Source: Association of Diabetes Care & Education Specialists and American Pharmacists Association
Key Takeaway: Developed by the Association for Diabetes Care and Education Specialists in partnership with APhA, this newly created Personal Continuous Glucose Monitoring (CGM) Implementation Playbook will help you implement a personal CGM program within your pharmacy practice.
This guide brings together fragmented information available from multiple sources to provide an inclusive and unbiased approach to implementation of Personal CGM into your practice, whatever its size. It includes a step-by-step approach to implementation, additional resources, and the latest research.
Download this free guide and start the process of incorporating this potentially game-changing tool for your patients living with diabetes.



February 26, 2021CGM Technology and Digital Health Article / PublicationSource: Association of Diabetes Care & Education Specialists and American Association of Nurse Practitioners
Key Takeaway: This toolkit provided by ADCES and AANP will help you implement a professional CGM program within your health system. Implementing a program within a healthcare setting offers many advantages, including: promotion of self-motivated, data-driven behavior change and improved clinical outcomes through alignment of medication with behavior change, resulting in lowered long-term healthcare costs for people with type 1 and type 2 diabetes.


LEARN MOREFebruary 10, 2021CGM Technology and Digital Health Article / Publication
Source: Diabetes Technology & Therapeutics
Key Takeaway: The role of real-time continuous glucose monitoring (rtCGM) is an essential component of telemedicine visits for people with diabetes. This observational study demonstrated that people with type 2 diabetes (T2D) participating in a virtual diabetes clinic can successfully insert and use Dexcom rtCGM without in-office training. The use of rtCGM was associated with a significant improvement in HbA1c at 10 months in those not meeting the ADA treatment target, independent of insulin use. In addition, there was a large shift in the percentage of participants meeting the HEDIS HbA1c target of <8.0% at follow-up; this may have important clinical and economic implications.
Chart: Percentage of Participants Achieving HEDIS HbA1c Treatment Target (HbA1c <8.0%) Before and After rtCGM Use

Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources


Change in HbA1c and Quality of Life with Real-time CGM Use by People with Insulin-Treated Diabetes in the Landmark Study
Source: Diabetes Technology and Therapeutics
Key Takeaway: The Landmark study demonstrated significant glycemic and QoL benefits for first time CGM use among individuals using intensive insulin therapy to manage either T1D or T2D. After approximately 12 weeks of Dexcom G6 use, participants had a mean absolute reduction in HbA1c levels of 1.1%, and more than half of those with initial HbA1c values >7% experienced absolute HbA1c reductions of >1%. The reduction in HbA1c observed in Landmark was similar for patients with T1D and T2D and was more pronounced for participants with higher baseline HbA1c, consistent with observations from the DIAMOND randomized controlled trial. Significant reductions in diabetes distress and hypoglycemic concerns were also observed. In the Landmark study, there was no standardized training or intervention at CGM initiation, suggesting that the glycemic benefits can be realized without formal instruction.
Changes in HbA1c according to baseline HbA1c level
Learn MoreFebruary 26, 2021CGM Technology and Digital Health Article / Publication

Source: Association of Diabetes Care & Education Specialists and American Pharmacists Association
Key Takeaway: Developed by the Association for Diabetes Care and Education Specialists in partnership with APhA, this newly created Personal Continuous Glucose Monitoring (CGM) Implementation Playbook will help you implement a personal CGM program within your pharmacy practice.
This guide brings together fragmented information available from multiple sources to provide an inclusive and unbiased approach to implementation of Personal CGM into your practice, whatever its size. It includes a step-by-step approach to implementation, additional resources, and the latest research.
Download this free guide and start the process of incorporating this potentially game-changing tool for your patients living with diabetes.



February 26, 2021CGM Technology and Digital Health Article / PublicationSource: Association of Diabetes Care & Education Specialists and American Association of Nurse Practitioners
Key Takeaway: This toolkit provided by ADCES and AANP will help you implement a professional CGM program within your health system. Implementing a program within a healthcare setting offers many advantages, including: promotion of self-motivated, data-driven behavior change and improved clinical outcomes through alignment of medication with behavior change, resulting in lowered long-term healthcare costs for people with type 1 and type 2 diabetes.


LEARN MOREFebruary 10, 2021CGM Technology and Digital Health Article / Publication
Source: Diabetes Technology & Therapeutics
Key Takeaway: The role of real-time continuous glucose monitoring (rtCGM) is an essential component of telemedicine visits for people with diabetes. This observational study demonstrated that people with type 2 diabetes (T2D) participating in a virtual diabetes clinic can successfully insert and use Dexcom rtCGM without in-office training. The use of rtCGM was associated with a significant improvement in HbA1c at 10 months in those not meeting the ADA treatment target, independent of insulin use. In addition, there was a large shift in the percentage of participants meeting the HEDIS HbA1c target of <8.0% at follow-up; this may have important clinical and economic implications.
Chart: Percentage of Participants Achieving HEDIS HbA1c Treatment Target (HbA1c <8.0%) Before and After rtCGM Use

Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources

Source: Association of Diabetes Care & Education Specialists and American Pharmacists Association
Key Takeaway: Developed by the Association for Diabetes Care and Education Specialists in partnership with APhA, this newly created Personal Continuous Glucose Monitoring (CGM) Implementation Playbook will help you implement a personal CGM program within your pharmacy practice.
This guide brings together fragmented information available from multiple sources to provide an inclusive and unbiased approach to implementation of Personal CGM into your practice, whatever its size. It includes a step-by-step approach to implementation, additional resources, and the latest research.
Download this free guide and start the process of incorporating this potentially game-changing tool for your patients living with diabetes.



February 26, 2021CGM Technology and Digital Health Article / PublicationSource: Association of Diabetes Care & Education Specialists and American Association of Nurse Practitioners
Key Takeaway: This toolkit provided by ADCES and AANP will help you implement a professional CGM program within your health system. Implementing a program within a healthcare setting offers many advantages, including: promotion of self-motivated, data-driven behavior change and improved clinical outcomes through alignment of medication with behavior change, resulting in lowered long-term healthcare costs for people with type 1 and type 2 diabetes.


LEARN MOREFebruary 10, 2021CGM Technology and Digital Health Article / Publication
Source: Diabetes Technology & Therapeutics
Key Takeaway: The role of real-time continuous glucose monitoring (rtCGM) is an essential component of telemedicine visits for people with diabetes. This observational study demonstrated that people with type 2 diabetes (T2D) participating in a virtual diabetes clinic can successfully insert and use Dexcom rtCGM without in-office training. The use of rtCGM was associated with a significant improvement in HbA1c at 10 months in those not meeting the ADA treatment target, independent of insulin use. In addition, there was a large shift in the percentage of participants meeting the HEDIS HbA1c target of <8.0% at follow-up; this may have important clinical and economic implications.
Chart: Percentage of Participants Achieving HEDIS HbA1c Treatment Target (HbA1c <8.0%) Before and After rtCGM Use

Source: Association of Diabetes Care & Education Specialists and American Association of Nurse Practitioners
Key Takeaway: This toolkit provided by ADCES and AANP will help you implement a professional CGM program within your health system. Implementing a program within a healthcare setting offers many advantages, including: promotion of self-motivated, data-driven behavior change and improved clinical outcomes through alignment of medication with behavior change, resulting in lowered long-term healthcare costs for people with type 1 and type 2 diabetes.


February 10, 2021CGM Technology and Digital Health Article / Publication
Source: Diabetes Technology & Therapeutics
Key Takeaway: The role of real-time continuous glucose monitoring (rtCGM) is an essential component of telemedicine visits for people with diabetes. This observational study demonstrated that people with type 2 diabetes (T2D) participating in a virtual diabetes clinic can successfully insert and use Dexcom rtCGM without in-office training. The use of rtCGM was associated with a significant improvement in HbA1c at 10 months in those not meeting the ADA treatment target, independent of insulin use. In addition, there was a large shift in the percentage of participants meeting the HEDIS HbA1c target of <8.0% at follow-up; this may have important clinical and economic implications.
Chart: Percentage of Participants Achieving HEDIS HbA1c Treatment Target (HbA1c <8.0%) Before and After rtCGM Use

Source: Diabetes Technology & Therapeutics
Key Takeaway: The role of real-time continuous glucose monitoring (rtCGM) is an essential component of telemedicine visits for people with diabetes. This observational study demonstrated that people with type 2 diabetes (T2D) participating in a virtual diabetes clinic can successfully insert and use Dexcom rtCGM without in-office training. The use of rtCGM was associated with a significant improvement in HbA1c at 10 months in those not meeting the ADA treatment target, independent of insulin use. In addition, there was a large shift in the percentage of participants meeting the HEDIS HbA1c target of <8.0% at follow-up; this may have important clinical and economic implications.
Chart: Percentage of Participants Achieving HEDIS HbA1c Treatment Target (HbA1c <8.0%) Before and After rtCGM Use