Change in HbA1c and Quality of Life with Real-time CGM Use by People with Insulin-Treated Diabetes in the Landmark Study
Source: Diabetes Technology and Therapeutics
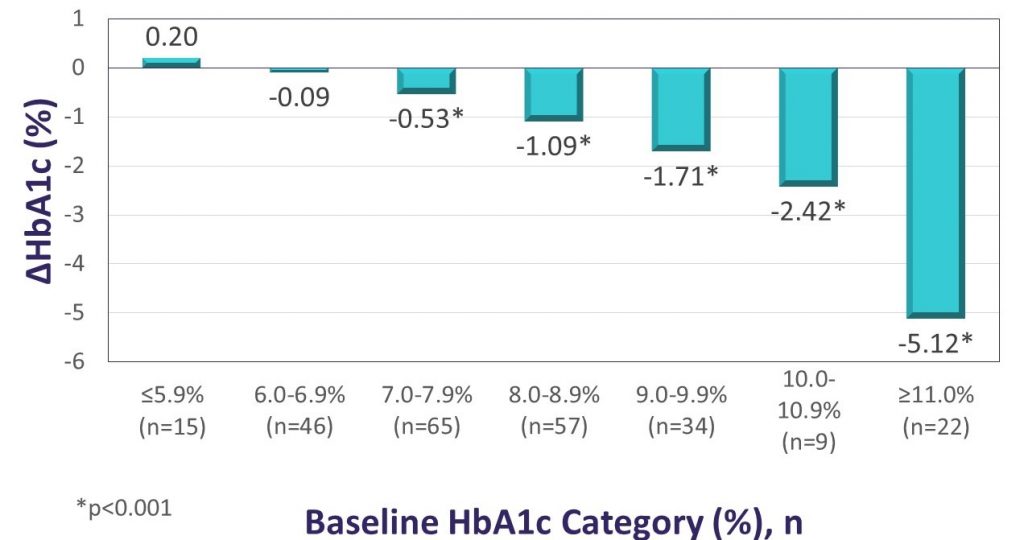
Key Takeaway: The Landmark study demonstrated significant glycemic and QoL benefits for first time CGM use among individuals using intensive insulin therapy to manage either T1D or T2D. After approximately 12 weeks of Dexcom G6 use, participants had a mean absolute reduction in HbA1c levels of 1.1%, and more than half of those with initial HbA1c values >7% experienced absolute HbA1c reductions of >1%. The reduction in HbA1c observed in Landmark was similar for patients with T1D and T2D and was more pronounced for participants with higher baseline HbA1c, consistent with observations from the DIAMOND randomized controlled trial. Significant reductions in diabetes distress and hypoglycemic concerns were also observed. In the Landmark study, there was no standardized training or intervention at CGM initiation, suggesting that the glycemic benefits can be realized without formal instruction.
Changes in HbA1c according to baseline HbA1c level
Learn More