CGM Innovations


The Benefit of the New Dexcom G7 with the New, Expanded CGM Medicare Coverage to Help Reduce Hypoglycemia
July 12, 2023CGM Technology and Digital Health Clinical Outcomes CGM Innovations / Webinar / Archive


CGM Innovations to Improve Diabetes Management: The Payer Value Proposition of a Next-Generation rtCGM System
February 17, 2023CGM Technology and Digital Health CGM Innovations / Webinar / Archive
Click here to download the summary from this live event or watch the webinar video archive below.
January 25, 2023CGM Technology and Digital Health CGM Innovations
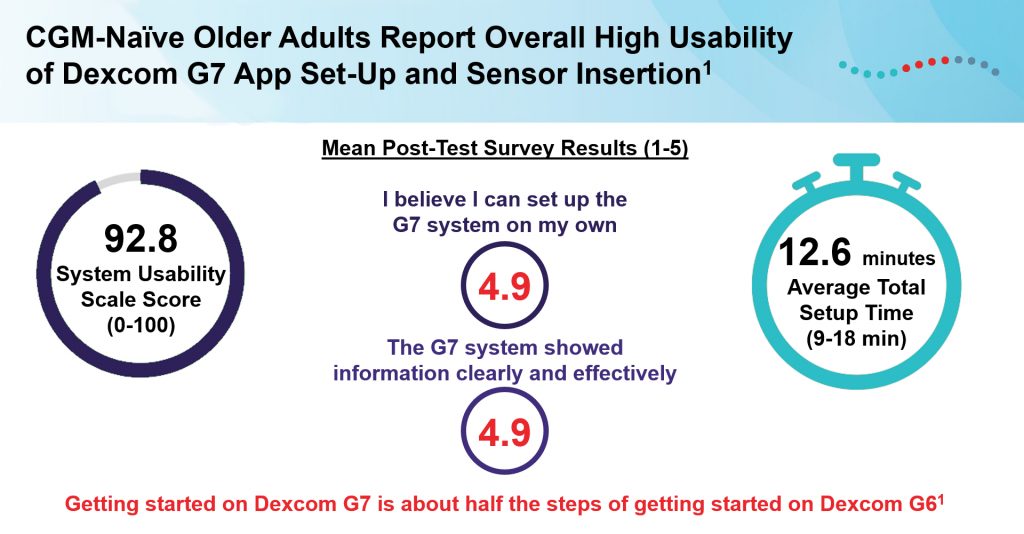
A task analysis and ease of use survey conducted among adults aged ≥65 years and certified diabetes care and education specialists (CDCESs) showed excellent useability associated with the new G7 RT-CGM system. Ease of use related to intradermal insertion and mobile app setup were assessed and compared to the fifth- and sixth-generation systems in the study, which recruited 10 older adults with no previous CGM experience and 10 CDCESs. The analysis revealed that approximately half as many tasks are needed to deploy the G7 system compared with the G6, resulting in excellent usability as assessed by older adults and CDCESs in the study, highlighted by a system usability score of 92.8. Cumulatively, these findings indicate a relatively low cognitive burden associated with the system compared with previous versions, simplifying the utilization of RT-CGM for older adults who are at higher risk for diabetes-related complications and stand to benefit from the implementation of diabetes technology. Managed care and payer decision makers may find the data presented in the study useful for informing coverage policy and criteria for RT-CGM in this vulnerable population of patients.
Psavko S, Katz N, Mirchi T, Green CR. Usability and Teachability of Continuous Glucose Monitoring Devices in Older Adults and Diabetes Educators: Task Analysis and Ease-of-Use Survey. JMIR Hum Factors. 2022;9(4):e42057.

1. Psavko S et al. JMIR Hum Factors 2022;9(4):42057 January 6, 2023Article / Publication / CGM Innovations
Visit the site to learn more about the value and use of CGM in the hospital setting.
Learn MoreJanuary 6, 2023Article / Publication / CGM Innovations
A comprehensive learning center for clinicians.
Learn More

New G7 Continuous Glucose Monitoring System Demonstrates Similar Accuracy and Superior Ease-of-Use Compared with Previous Versions
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In an analysis of available data, accuracy metrics from preapproval trials of the G5, G6, and G7 real-time continuous glucose monitoring (RT-CGM) systems were compared after propensity score adjustments were applied to balance baseline demographic characteristics. Metrics included mean absolute relative differences (MARD) between CGM and YSI values (from the YSI, Inc., 2300 Stat Plus system) and the proportion of CGM values within 20% or 20 mg/dL of the YSI values (“%20/20”). Ease-of-use was also evaluated by formal task analysis in the study. Accuracy performance of the G7 sensors, whether placed on the arm or abdomen, was similar to that of abdomen-placed G5 and G6 sensors, and egregious errors were rare with all three systems. Based on the formal task analysis, the authors concluded that simplification of the sensor insertion process should result in G7 being even easier to learn and several software improvements may contribute to better glycemic outcomes. Payer professionals may well find confidence in the comparative usability data for the latest RT-CGM system versus previous versions of the technology when considering updates to coverage policies.
Welsh JB, Psavko S, Zhang X, Gao P, Balo AK. Comparisons of Fifth-, Sixth-, and Seventh-Generation Continuous Glucose Monitoring Systems. J Diabetes Sci Technol. 2022:19322968221099879.
Learn More

In Children and Adolescents with Type 1 Diabetes, G7 Continuous Glucose Monitoring System Maintains Accuracy Across Wear Days, Glucose Ranges, and Rates of Glucose Change
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In children and adolescents with type 1 diabetes (T1D), the G7 real-time continuous glucose monitoring (RT-CGM) system demonstrated accuracy across wear days, glucose ranges, and rates of glucose change. Over 10.5 days, RT-CGM data from 28 participants aged 2 to 6 years and 127 participants aged 7 to 17 were collected and compared with blood glucose measurements. In young children aged 2 to 6, overall MARD and %20/20 agreement rates were 9.3% and 91.5%, respectively. In older children and adolescents aged 7 to 17 years, arm- and abdomen-placed G7 sensor MARD values were 8.1% and 9.0%, respectively, and overall %20/20 values were 95.3% and 92.9%, respectively. These results are consistent with those of the G6 system in children and adolescents with T1D and with an earlier study of the G5 system that evaluated accuracy at different insertion sites. Compared with the G6 RT-CGM system, the G7 has a shorter warm-up period (27 minutes vs 2 hours), making it possible for youth and caregivers of young children to obtain glucose data more quickly for diabetes management decisions. According to the authors, while day 1 accuracy tends to be lower across CGM devices, the accuracy of the G7—coupled with its shorter warm-up period—should improve the sensor experience in young users. The G7 RT-CGM was recently FDA approved for use in all individuals with diabetes aged 2 years and older, and having data specific to use in children and adolescents can be of value for managed care and payer professionals in developing coverage policies.
Laffel LM, Bailey TS, Christiansen MP, Reid JL, Beck SE. Accuracy of a Seventh-Generation Continuous Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. J Diabetes Sci Technol. 2022:19322968221091816.
Learn More

G7 Real-Time Continuous Glucose Monitoring System Demonstrates Accurate Glucose Readings and No Serious Adverse Events in Adults with Type 1 and Type 2 Diabetes
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
The accuracy and safety of the G7 real-time continuous glucose monitoring (RT-CGM) system was demonstrated over 10.5 days of use in adults with diabetes. In the study, adults with either type 1 or type 2 diabetes—either on intensive insulin therapy or not—wore G7 sensors concurrently on the upper arm and abdomen. Individuals were seen in clinic on days 1 or 2, 4 or 7, and on the second half of day 10 or the first half of day 11 for frequent comparisons with comparator blood glucose measurements obtained with the YSI 2300 Stat Plus glucose analyzer. After analyzing data from 316 participants (619 sensors, 77,774 matched pairs), overall mean absolute relative differences (MARD) between RT-CGM and YSI values for arm- and abdomen-placed sensors were 8.2% and 9.1%, respectively. In-clinic glucose manipulations and frequent blood glucose sampling confirmed accurate readings during euglycemia, hypoglycemia, and hyperglycemia (reflected as time in range [TIR], time below range [TBR], and time above range [TAR]), as well as during rapid glucose concentration change. Even at the highest rates of glucose concentration change, MARD values <10% were observed for arm-placed sensors and were <10.5% for abdomen-placed sensors. Offering a smaller size and added features, such as sensor/transmitter integration with a simplified insertion process, the G7 also demonstrated a favorable safety profile, with no serious adverse events reported during the study. With the recent FDA approval of the G7 RT-CGM system, payer professionals may well find value in the accuracy and safety data from this prospective multicenter single-arm study when they are developing coverage criteria.
Garg SK, et al. Accuracy and Safety of Dexcom G7 Continuous Glucose Monitoring in Adults with Diabetes. Diabetes Technol Ther. 2022;24(6):373-380.
Learn More

National Institute for Health and Care Excellence (NICE) Medtech Innovation Briefing for Dexcom G6 Real-Time CGM
November 3, 2020Economic Outcomes Article / Publication / CGM Innovations
Source:
Key Takeaway: The intended place in therapy is as an alternative to routine blood glucose monitoring in people (over 2 years old), including pregnant women, with type 1 or type 2 diabetes, who use multiple daily insulin injections or use insulin pumps and are self-managing their diabetes. Dexcom G6 could reduce costs and would benefit the healthcare system by improving long-term outcomes, reducing the need for intensive treatment and, in the short term, reducing severe hypoglycaemic events leading to hospital admissions. Remote care may reduce the need for hospital visits.
Learn More- 1
- 1-9 of 9 results
Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources


The Benefit of the New Dexcom G7 with the New, Expanded CGM Medicare Coverage to Help Reduce Hypoglycemia


CGM Innovations to Improve Diabetes Management: The Payer Value Proposition of a Next-Generation rtCGM System
February 17, 2023CGM Technology and Digital Health CGM Innovations / Webinar / Archive
Click here to download the summary from this live event or watch the webinar video archive below.
January 25, 2023CGM Technology and Digital Health CGM Innovations
A task analysis and ease of use survey conducted among adults aged ≥65 years and certified diabetes care and education specialists (CDCESs) showed excellent useability associated with the new G7 RT-CGM system. Ease of use related to intradermal insertion and mobile app setup were assessed and compared to the fifth- and sixth-generation systems in the study, which recruited 10 older adults with no previous CGM experience and 10 CDCESs. The analysis revealed that approximately half as many tasks are needed to deploy the G7 system compared with the G6, resulting in excellent usability as assessed by older adults and CDCESs in the study, highlighted by a system usability score of 92.8. Cumulatively, these findings indicate a relatively low cognitive burden associated with the system compared with previous versions, simplifying the utilization of RT-CGM for older adults who are at higher risk for diabetes-related complications and stand to benefit from the implementation of diabetes technology. Managed care and payer decision makers may find the data presented in the study useful for informing coverage policy and criteria for RT-CGM in this vulnerable population of patients.
Psavko S, Katz N, Mirchi T, Green CR. Usability and Teachability of Continuous Glucose Monitoring Devices in Older Adults and Diabetes Educators: Task Analysis and Ease-of-Use Survey. JMIR Hum Factors. 2022;9(4):e42057.

1. Psavko S et al. JMIR Hum Factors 2022;9(4):42057 January 6, 2023Article / Publication / CGM Innovations
Visit the site to learn more about the value and use of CGM in the hospital setting.
Learn MoreJanuary 6, 2023Article / Publication / CGM Innovations
A comprehensive learning center for clinicians.
Learn More

New G7 Continuous Glucose Monitoring System Demonstrates Similar Accuracy and Superior Ease-of-Use Compared with Previous Versions
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In an analysis of available data, accuracy metrics from preapproval trials of the G5, G6, and G7 real-time continuous glucose monitoring (RT-CGM) systems were compared after propensity score adjustments were applied to balance baseline demographic characteristics. Metrics included mean absolute relative differences (MARD) between CGM and YSI values (from the YSI, Inc., 2300 Stat Plus system) and the proportion of CGM values within 20% or 20 mg/dL of the YSI values (“%20/20”). Ease-of-use was also evaluated by formal task analysis in the study. Accuracy performance of the G7 sensors, whether placed on the arm or abdomen, was similar to that of abdomen-placed G5 and G6 sensors, and egregious errors were rare with all three systems. Based on the formal task analysis, the authors concluded that simplification of the sensor insertion process should result in G7 being even easier to learn and several software improvements may contribute to better glycemic outcomes. Payer professionals may well find confidence in the comparative usability data for the latest RT-CGM system versus previous versions of the technology when considering updates to coverage policies.
Welsh JB, Psavko S, Zhang X, Gao P, Balo AK. Comparisons of Fifth-, Sixth-, and Seventh-Generation Continuous Glucose Monitoring Systems. J Diabetes Sci Technol. 2022:19322968221099879.
Learn More

In Children and Adolescents with Type 1 Diabetes, G7 Continuous Glucose Monitoring System Maintains Accuracy Across Wear Days, Glucose Ranges, and Rates of Glucose Change
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In children and adolescents with type 1 diabetes (T1D), the G7 real-time continuous glucose monitoring (RT-CGM) system demonstrated accuracy across wear days, glucose ranges, and rates of glucose change. Over 10.5 days, RT-CGM data from 28 participants aged 2 to 6 years and 127 participants aged 7 to 17 were collected and compared with blood glucose measurements. In young children aged 2 to 6, overall MARD and %20/20 agreement rates were 9.3% and 91.5%, respectively. In older children and adolescents aged 7 to 17 years, arm- and abdomen-placed G7 sensor MARD values were 8.1% and 9.0%, respectively, and overall %20/20 values were 95.3% and 92.9%, respectively. These results are consistent with those of the G6 system in children and adolescents with T1D and with an earlier study of the G5 system that evaluated accuracy at different insertion sites. Compared with the G6 RT-CGM system, the G7 has a shorter warm-up period (27 minutes vs 2 hours), making it possible for youth and caregivers of young children to obtain glucose data more quickly for diabetes management decisions. According to the authors, while day 1 accuracy tends to be lower across CGM devices, the accuracy of the G7—coupled with its shorter warm-up period—should improve the sensor experience in young users. The G7 RT-CGM was recently FDA approved for use in all individuals with diabetes aged 2 years and older, and having data specific to use in children and adolescents can be of value for managed care and payer professionals in developing coverage policies.
Laffel LM, Bailey TS, Christiansen MP, Reid JL, Beck SE. Accuracy of a Seventh-Generation Continuous Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. J Diabetes Sci Technol. 2022:19322968221091816.
Learn More

G7 Real-Time Continuous Glucose Monitoring System Demonstrates Accurate Glucose Readings and No Serious Adverse Events in Adults with Type 1 and Type 2 Diabetes
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
The accuracy and safety of the G7 real-time continuous glucose monitoring (RT-CGM) system was demonstrated over 10.5 days of use in adults with diabetes. In the study, adults with either type 1 or type 2 diabetes—either on intensive insulin therapy or not—wore G7 sensors concurrently on the upper arm and abdomen. Individuals were seen in clinic on days 1 or 2, 4 or 7, and on the second half of day 10 or the first half of day 11 for frequent comparisons with comparator blood glucose measurements obtained with the YSI 2300 Stat Plus glucose analyzer. After analyzing data from 316 participants (619 sensors, 77,774 matched pairs), overall mean absolute relative differences (MARD) between RT-CGM and YSI values for arm- and abdomen-placed sensors were 8.2% and 9.1%, respectively. In-clinic glucose manipulations and frequent blood glucose sampling confirmed accurate readings during euglycemia, hypoglycemia, and hyperglycemia (reflected as time in range [TIR], time below range [TBR], and time above range [TAR]), as well as during rapid glucose concentration change. Even at the highest rates of glucose concentration change, MARD values <10% were observed for arm-placed sensors and were <10.5% for abdomen-placed sensors. Offering a smaller size and added features, such as sensor/transmitter integration with a simplified insertion process, the G7 also demonstrated a favorable safety profile, with no serious adverse events reported during the study. With the recent FDA approval of the G7 RT-CGM system, payer professionals may well find value in the accuracy and safety data from this prospective multicenter single-arm study when they are developing coverage criteria.
Garg SK, et al. Accuracy and Safety of Dexcom G7 Continuous Glucose Monitoring in Adults with Diabetes. Diabetes Technol Ther. 2022;24(6):373-380.
Learn More

National Institute for Health and Care Excellence (NICE) Medtech Innovation Briefing for Dexcom G6 Real-Time CGM
November 3, 2020Economic Outcomes Article / Publication / CGM Innovations
Source:
Key Takeaway: The intended place in therapy is as an alternative to routine blood glucose monitoring in people (over 2 years old), including pregnant women, with type 1 or type 2 diabetes, who use multiple daily insulin injections or use insulin pumps and are self-managing their diabetes. Dexcom G6 could reduce costs and would benefit the healthcare system by improving long-term outcomes, reducing the need for intensive treatment and, in the short term, reducing severe hypoglycaemic events leading to hospital admissions. Remote care may reduce the need for hospital visits.
Learn More- 1
- 1-9 of 9 results
Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources


CGM Innovations to Improve Diabetes Management: The Payer Value Proposition of a Next-Generation rtCGM System
Click here to download the summary from this live event or watch the webinar video archive below.
January 25, 2023CGM Technology and Digital Health CGM Innovations
A task analysis and ease of use survey conducted among adults aged ≥65 years and certified diabetes care and education specialists (CDCESs) showed excellent useability associated with the new G7 RT-CGM system. Ease of use related to intradermal insertion and mobile app setup were assessed and compared to the fifth- and sixth-generation systems in the study, which recruited 10 older adults with no previous CGM experience and 10 CDCESs. The analysis revealed that approximately half as many tasks are needed to deploy the G7 system compared with the G6, resulting in excellent usability as assessed by older adults and CDCESs in the study, highlighted by a system usability score of 92.8. Cumulatively, these findings indicate a relatively low cognitive burden associated with the system compared with previous versions, simplifying the utilization of RT-CGM for older adults who are at higher risk for diabetes-related complications and stand to benefit from the implementation of diabetes technology. Managed care and payer decision makers may find the data presented in the study useful for informing coverage policy and criteria for RT-CGM in this vulnerable population of patients.
Psavko S, Katz N, Mirchi T, Green CR. Usability and Teachability of Continuous Glucose Monitoring Devices in Older Adults and Diabetes Educators: Task Analysis and Ease-of-Use Survey. JMIR Hum Factors. 2022;9(4):e42057.

1. Psavko S et al. JMIR Hum Factors 2022;9(4):42057 January 6, 2023Article / Publication / CGM Innovations
Visit the site to learn more about the value and use of CGM in the hospital setting.
Learn MoreJanuary 6, 2023Article / Publication / CGM Innovations
A comprehensive learning center for clinicians.
Learn More

New G7 Continuous Glucose Monitoring System Demonstrates Similar Accuracy and Superior Ease-of-Use Compared with Previous Versions
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In an analysis of available data, accuracy metrics from preapproval trials of the G5, G6, and G7 real-time continuous glucose monitoring (RT-CGM) systems were compared after propensity score adjustments were applied to balance baseline demographic characteristics. Metrics included mean absolute relative differences (MARD) between CGM and YSI values (from the YSI, Inc., 2300 Stat Plus system) and the proportion of CGM values within 20% or 20 mg/dL of the YSI values (“%20/20”). Ease-of-use was also evaluated by formal task analysis in the study. Accuracy performance of the G7 sensors, whether placed on the arm or abdomen, was similar to that of abdomen-placed G5 and G6 sensors, and egregious errors were rare with all three systems. Based on the formal task analysis, the authors concluded that simplification of the sensor insertion process should result in G7 being even easier to learn and several software improvements may contribute to better glycemic outcomes. Payer professionals may well find confidence in the comparative usability data for the latest RT-CGM system versus previous versions of the technology when considering updates to coverage policies.
Welsh JB, Psavko S, Zhang X, Gao P, Balo AK. Comparisons of Fifth-, Sixth-, and Seventh-Generation Continuous Glucose Monitoring Systems. J Diabetes Sci Technol. 2022:19322968221099879.
Learn More

In Children and Adolescents with Type 1 Diabetes, G7 Continuous Glucose Monitoring System Maintains Accuracy Across Wear Days, Glucose Ranges, and Rates of Glucose Change
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In children and adolescents with type 1 diabetes (T1D), the G7 real-time continuous glucose monitoring (RT-CGM) system demonstrated accuracy across wear days, glucose ranges, and rates of glucose change. Over 10.5 days, RT-CGM data from 28 participants aged 2 to 6 years and 127 participants aged 7 to 17 were collected and compared with blood glucose measurements. In young children aged 2 to 6, overall MARD and %20/20 agreement rates were 9.3% and 91.5%, respectively. In older children and adolescents aged 7 to 17 years, arm- and abdomen-placed G7 sensor MARD values were 8.1% and 9.0%, respectively, and overall %20/20 values were 95.3% and 92.9%, respectively. These results are consistent with those of the G6 system in children and adolescents with T1D and with an earlier study of the G5 system that evaluated accuracy at different insertion sites. Compared with the G6 RT-CGM system, the G7 has a shorter warm-up period (27 minutes vs 2 hours), making it possible for youth and caregivers of young children to obtain glucose data more quickly for diabetes management decisions. According to the authors, while day 1 accuracy tends to be lower across CGM devices, the accuracy of the G7—coupled with its shorter warm-up period—should improve the sensor experience in young users. The G7 RT-CGM was recently FDA approved for use in all individuals with diabetes aged 2 years and older, and having data specific to use in children and adolescents can be of value for managed care and payer professionals in developing coverage policies.
Laffel LM, Bailey TS, Christiansen MP, Reid JL, Beck SE. Accuracy of a Seventh-Generation Continuous Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. J Diabetes Sci Technol. 2022:19322968221091816.
Learn More

G7 Real-Time Continuous Glucose Monitoring System Demonstrates Accurate Glucose Readings and No Serious Adverse Events in Adults with Type 1 and Type 2 Diabetes
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
The accuracy and safety of the G7 real-time continuous glucose monitoring (RT-CGM) system was demonstrated over 10.5 days of use in adults with diabetes. In the study, adults with either type 1 or type 2 diabetes—either on intensive insulin therapy or not—wore G7 sensors concurrently on the upper arm and abdomen. Individuals were seen in clinic on days 1 or 2, 4 or 7, and on the second half of day 10 or the first half of day 11 for frequent comparisons with comparator blood glucose measurements obtained with the YSI 2300 Stat Plus glucose analyzer. After analyzing data from 316 participants (619 sensors, 77,774 matched pairs), overall mean absolute relative differences (MARD) between RT-CGM and YSI values for arm- and abdomen-placed sensors were 8.2% and 9.1%, respectively. In-clinic glucose manipulations and frequent blood glucose sampling confirmed accurate readings during euglycemia, hypoglycemia, and hyperglycemia (reflected as time in range [TIR], time below range [TBR], and time above range [TAR]), as well as during rapid glucose concentration change. Even at the highest rates of glucose concentration change, MARD values <10% were observed for arm-placed sensors and were <10.5% for abdomen-placed sensors. Offering a smaller size and added features, such as sensor/transmitter integration with a simplified insertion process, the G7 also demonstrated a favorable safety profile, with no serious adverse events reported during the study. With the recent FDA approval of the G7 RT-CGM system, payer professionals may well find value in the accuracy and safety data from this prospective multicenter single-arm study when they are developing coverage criteria.
Garg SK, et al. Accuracy and Safety of Dexcom G7 Continuous Glucose Monitoring in Adults with Diabetes. Diabetes Technol Ther. 2022;24(6):373-380.
Learn More

National Institute for Health and Care Excellence (NICE) Medtech Innovation Briefing for Dexcom G6 Real-Time CGM
November 3, 2020Economic Outcomes Article / Publication / CGM Innovations
Source:
Key Takeaway: The intended place in therapy is as an alternative to routine blood glucose monitoring in people (over 2 years old), including pregnant women, with type 1 or type 2 diabetes, who use multiple daily insulin injections or use insulin pumps and are self-managing their diabetes. Dexcom G6 could reduce costs and would benefit the healthcare system by improving long-term outcomes, reducing the need for intensive treatment and, in the short term, reducing severe hypoglycaemic events leading to hospital admissions. Remote care may reduce the need for hospital visits.
Learn More- 1
- 1-9 of 9 results
Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources
A task analysis and ease of use survey conducted among adults aged ≥65 years and certified diabetes care and education specialists (CDCESs) showed excellent useability associated with the new G7 RT-CGM system. Ease of use related to intradermal insertion and mobile app setup were assessed and compared to the fifth- and sixth-generation systems in the study, which recruited 10 older adults with no previous CGM experience and 10 CDCESs. The analysis revealed that approximately half as many tasks are needed to deploy the G7 system compared with the G6, resulting in excellent usability as assessed by older adults and CDCESs in the study, highlighted by a system usability score of 92.8. Cumulatively, these findings indicate a relatively low cognitive burden associated with the system compared with previous versions, simplifying the utilization of RT-CGM for older adults who are at higher risk for diabetes-related complications and stand to benefit from the implementation of diabetes technology. Managed care and payer decision makers may find the data presented in the study useful for informing coverage policy and criteria for RT-CGM in this vulnerable population of patients.
Psavko S, Katz N, Mirchi T, Green CR. Usability and Teachability of Continuous Glucose Monitoring Devices in Older Adults and Diabetes Educators: Task Analysis and Ease-of-Use Survey. JMIR Hum Factors. 2022;9(4):e42057.
January 6, 2023Article / Publication / CGM Innovations
Visit the site to learn more about the value and use of CGM in the hospital setting.
Learn MoreJanuary 6, 2023Article / Publication / CGM Innovations
A comprehensive learning center for clinicians.
Learn More

New G7 Continuous Glucose Monitoring System Demonstrates Similar Accuracy and Superior Ease-of-Use Compared with Previous Versions
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In an analysis of available data, accuracy metrics from preapproval trials of the G5, G6, and G7 real-time continuous glucose monitoring (RT-CGM) systems were compared after propensity score adjustments were applied to balance baseline demographic characteristics. Metrics included mean absolute relative differences (MARD) between CGM and YSI values (from the YSI, Inc., 2300 Stat Plus system) and the proportion of CGM values within 20% or 20 mg/dL of the YSI values (“%20/20”). Ease-of-use was also evaluated by formal task analysis in the study. Accuracy performance of the G7 sensors, whether placed on the arm or abdomen, was similar to that of abdomen-placed G5 and G6 sensors, and egregious errors were rare with all three systems. Based on the formal task analysis, the authors concluded that simplification of the sensor insertion process should result in G7 being even easier to learn and several software improvements may contribute to better glycemic outcomes. Payer professionals may well find confidence in the comparative usability data for the latest RT-CGM system versus previous versions of the technology when considering updates to coverage policies.
Welsh JB, Psavko S, Zhang X, Gao P, Balo AK. Comparisons of Fifth-, Sixth-, and Seventh-Generation Continuous Glucose Monitoring Systems. J Diabetes Sci Technol. 2022:19322968221099879.
Learn More

In Children and Adolescents with Type 1 Diabetes, G7 Continuous Glucose Monitoring System Maintains Accuracy Across Wear Days, Glucose Ranges, and Rates of Glucose Change
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In children and adolescents with type 1 diabetes (T1D), the G7 real-time continuous glucose monitoring (RT-CGM) system demonstrated accuracy across wear days, glucose ranges, and rates of glucose change. Over 10.5 days, RT-CGM data from 28 participants aged 2 to 6 years and 127 participants aged 7 to 17 were collected and compared with blood glucose measurements. In young children aged 2 to 6, overall MARD and %20/20 agreement rates were 9.3% and 91.5%, respectively. In older children and adolescents aged 7 to 17 years, arm- and abdomen-placed G7 sensor MARD values were 8.1% and 9.0%, respectively, and overall %20/20 values were 95.3% and 92.9%, respectively. These results are consistent with those of the G6 system in children and adolescents with T1D and with an earlier study of the G5 system that evaluated accuracy at different insertion sites. Compared with the G6 RT-CGM system, the G7 has a shorter warm-up period (27 minutes vs 2 hours), making it possible for youth and caregivers of young children to obtain glucose data more quickly for diabetes management decisions. According to the authors, while day 1 accuracy tends to be lower across CGM devices, the accuracy of the G7—coupled with its shorter warm-up period—should improve the sensor experience in young users. The G7 RT-CGM was recently FDA approved for use in all individuals with diabetes aged 2 years and older, and having data specific to use in children and adolescents can be of value for managed care and payer professionals in developing coverage policies.
Laffel LM, Bailey TS, Christiansen MP, Reid JL, Beck SE. Accuracy of a Seventh-Generation Continuous Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. J Diabetes Sci Technol. 2022:19322968221091816.
Learn More

G7 Real-Time Continuous Glucose Monitoring System Demonstrates Accurate Glucose Readings and No Serious Adverse Events in Adults with Type 1 and Type 2 Diabetes
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
The accuracy and safety of the G7 real-time continuous glucose monitoring (RT-CGM) system was demonstrated over 10.5 days of use in adults with diabetes. In the study, adults with either type 1 or type 2 diabetes—either on intensive insulin therapy or not—wore G7 sensors concurrently on the upper arm and abdomen. Individuals were seen in clinic on days 1 or 2, 4 or 7, and on the second half of day 10 or the first half of day 11 for frequent comparisons with comparator blood glucose measurements obtained with the YSI 2300 Stat Plus glucose analyzer. After analyzing data from 316 participants (619 sensors, 77,774 matched pairs), overall mean absolute relative differences (MARD) between RT-CGM and YSI values for arm- and abdomen-placed sensors were 8.2% and 9.1%, respectively. In-clinic glucose manipulations and frequent blood glucose sampling confirmed accurate readings during euglycemia, hypoglycemia, and hyperglycemia (reflected as time in range [TIR], time below range [TBR], and time above range [TAR]), as well as during rapid glucose concentration change. Even at the highest rates of glucose concentration change, MARD values <10% were observed for arm-placed sensors and were <10.5% for abdomen-placed sensors. Offering a smaller size and added features, such as sensor/transmitter integration with a simplified insertion process, the G7 also demonstrated a favorable safety profile, with no serious adverse events reported during the study. With the recent FDA approval of the G7 RT-CGM system, payer professionals may well find value in the accuracy and safety data from this prospective multicenter single-arm study when they are developing coverage criteria.
Garg SK, et al. Accuracy and Safety of Dexcom G7 Continuous Glucose Monitoring in Adults with Diabetes. Diabetes Technol Ther. 2022;24(6):373-380.
Learn More

National Institute for Health and Care Excellence (NICE) Medtech Innovation Briefing for Dexcom G6 Real-Time CGM
November 3, 2020Economic Outcomes Article / Publication / CGM Innovations
Source:
Key Takeaway: The intended place in therapy is as an alternative to routine blood glucose monitoring in people (over 2 years old), including pregnant women, with type 1 or type 2 diabetes, who use multiple daily insulin injections or use insulin pumps and are self-managing their diabetes. Dexcom G6 could reduce costs and would benefit the healthcare system by improving long-term outcomes, reducing the need for intensive treatment and, in the short term, reducing severe hypoglycaemic events leading to hospital admissions. Remote care may reduce the need for hospital visits.
Learn More- 1
- 1-9 of 9 results
Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources
Visit the site to learn more about the value and use of CGM in the hospital setting.
Learn MoreJanuary 6, 2023Article / Publication / CGM Innovations
A comprehensive learning center for clinicians.
Learn More

New G7 Continuous Glucose Monitoring System Demonstrates Similar Accuracy and Superior Ease-of-Use Compared with Previous Versions
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In an analysis of available data, accuracy metrics from preapproval trials of the G5, G6, and G7 real-time continuous glucose monitoring (RT-CGM) systems were compared after propensity score adjustments were applied to balance baseline demographic characteristics. Metrics included mean absolute relative differences (MARD) between CGM and YSI values (from the YSI, Inc., 2300 Stat Plus system) and the proportion of CGM values within 20% or 20 mg/dL of the YSI values (“%20/20”). Ease-of-use was also evaluated by formal task analysis in the study. Accuracy performance of the G7 sensors, whether placed on the arm or abdomen, was similar to that of abdomen-placed G5 and G6 sensors, and egregious errors were rare with all three systems. Based on the formal task analysis, the authors concluded that simplification of the sensor insertion process should result in G7 being even easier to learn and several software improvements may contribute to better glycemic outcomes. Payer professionals may well find confidence in the comparative usability data for the latest RT-CGM system versus previous versions of the technology when considering updates to coverage policies.
Welsh JB, Psavko S, Zhang X, Gao P, Balo AK. Comparisons of Fifth-, Sixth-, and Seventh-Generation Continuous Glucose Monitoring Systems. J Diabetes Sci Technol. 2022:19322968221099879.
Learn More

In Children and Adolescents with Type 1 Diabetes, G7 Continuous Glucose Monitoring System Maintains Accuracy Across Wear Days, Glucose Ranges, and Rates of Glucose Change
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In children and adolescents with type 1 diabetes (T1D), the G7 real-time continuous glucose monitoring (RT-CGM) system demonstrated accuracy across wear days, glucose ranges, and rates of glucose change. Over 10.5 days, RT-CGM data from 28 participants aged 2 to 6 years and 127 participants aged 7 to 17 were collected and compared with blood glucose measurements. In young children aged 2 to 6, overall MARD and %20/20 agreement rates were 9.3% and 91.5%, respectively. In older children and adolescents aged 7 to 17 years, arm- and abdomen-placed G7 sensor MARD values were 8.1% and 9.0%, respectively, and overall %20/20 values were 95.3% and 92.9%, respectively. These results are consistent with those of the G6 system in children and adolescents with T1D and with an earlier study of the G5 system that evaluated accuracy at different insertion sites. Compared with the G6 RT-CGM system, the G7 has a shorter warm-up period (27 minutes vs 2 hours), making it possible for youth and caregivers of young children to obtain glucose data more quickly for diabetes management decisions. According to the authors, while day 1 accuracy tends to be lower across CGM devices, the accuracy of the G7—coupled with its shorter warm-up period—should improve the sensor experience in young users. The G7 RT-CGM was recently FDA approved for use in all individuals with diabetes aged 2 years and older, and having data specific to use in children and adolescents can be of value for managed care and payer professionals in developing coverage policies.
Laffel LM, Bailey TS, Christiansen MP, Reid JL, Beck SE. Accuracy of a Seventh-Generation Continuous Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. J Diabetes Sci Technol. 2022:19322968221091816.
Learn More

G7 Real-Time Continuous Glucose Monitoring System Demonstrates Accurate Glucose Readings and No Serious Adverse Events in Adults with Type 1 and Type 2 Diabetes
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
The accuracy and safety of the G7 real-time continuous glucose monitoring (RT-CGM) system was demonstrated over 10.5 days of use in adults with diabetes. In the study, adults with either type 1 or type 2 diabetes—either on intensive insulin therapy or not—wore G7 sensors concurrently on the upper arm and abdomen. Individuals were seen in clinic on days 1 or 2, 4 or 7, and on the second half of day 10 or the first half of day 11 for frequent comparisons with comparator blood glucose measurements obtained with the YSI 2300 Stat Plus glucose analyzer. After analyzing data from 316 participants (619 sensors, 77,774 matched pairs), overall mean absolute relative differences (MARD) between RT-CGM and YSI values for arm- and abdomen-placed sensors were 8.2% and 9.1%, respectively. In-clinic glucose manipulations and frequent blood glucose sampling confirmed accurate readings during euglycemia, hypoglycemia, and hyperglycemia (reflected as time in range [TIR], time below range [TBR], and time above range [TAR]), as well as during rapid glucose concentration change. Even at the highest rates of glucose concentration change, MARD values <10% were observed for arm-placed sensors and were <10.5% for abdomen-placed sensors. Offering a smaller size and added features, such as sensor/transmitter integration with a simplified insertion process, the G7 also demonstrated a favorable safety profile, with no serious adverse events reported during the study. With the recent FDA approval of the G7 RT-CGM system, payer professionals may well find value in the accuracy and safety data from this prospective multicenter single-arm study when they are developing coverage criteria.
Garg SK, et al. Accuracy and Safety of Dexcom G7 Continuous Glucose Monitoring in Adults with Diabetes. Diabetes Technol Ther. 2022;24(6):373-380.
Learn More

National Institute for Health and Care Excellence (NICE) Medtech Innovation Briefing for Dexcom G6 Real-Time CGM
November 3, 2020Economic Outcomes Article / Publication / CGM Innovations
Source:
Key Takeaway: The intended place in therapy is as an alternative to routine blood glucose monitoring in people (over 2 years old), including pregnant women, with type 1 or type 2 diabetes, who use multiple daily insulin injections or use insulin pumps and are self-managing their diabetes. Dexcom G6 could reduce costs and would benefit the healthcare system by improving long-term outcomes, reducing the need for intensive treatment and, in the short term, reducing severe hypoglycaemic events leading to hospital admissions. Remote care may reduce the need for hospital visits.
Learn More- 1
- 1-9 of 9 results
Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources
A comprehensive learning center for clinicians.
Learn More

New G7 Continuous Glucose Monitoring System Demonstrates Similar Accuracy and Superior Ease-of-Use Compared with Previous Versions
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In an analysis of available data, accuracy metrics from preapproval trials of the G5, G6, and G7 real-time continuous glucose monitoring (RT-CGM) systems were compared after propensity score adjustments were applied to balance baseline demographic characteristics. Metrics included mean absolute relative differences (MARD) between CGM and YSI values (from the YSI, Inc., 2300 Stat Plus system) and the proportion of CGM values within 20% or 20 mg/dL of the YSI values (“%20/20”). Ease-of-use was also evaluated by formal task analysis in the study. Accuracy performance of the G7 sensors, whether placed on the arm or abdomen, was similar to that of abdomen-placed G5 and G6 sensors, and egregious errors were rare with all three systems. Based on the formal task analysis, the authors concluded that simplification of the sensor insertion process should result in G7 being even easier to learn and several software improvements may contribute to better glycemic outcomes. Payer professionals may well find confidence in the comparative usability data for the latest RT-CGM system versus previous versions of the technology when considering updates to coverage policies.
Welsh JB, Psavko S, Zhang X, Gao P, Balo AK. Comparisons of Fifth-, Sixth-, and Seventh-Generation Continuous Glucose Monitoring Systems. J Diabetes Sci Technol. 2022:19322968221099879.
Learn More

In Children and Adolescents with Type 1 Diabetes, G7 Continuous Glucose Monitoring System Maintains Accuracy Across Wear Days, Glucose Ranges, and Rates of Glucose Change
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In children and adolescents with type 1 diabetes (T1D), the G7 real-time continuous glucose monitoring (RT-CGM) system demonstrated accuracy across wear days, glucose ranges, and rates of glucose change. Over 10.5 days, RT-CGM data from 28 participants aged 2 to 6 years and 127 participants aged 7 to 17 were collected and compared with blood glucose measurements. In young children aged 2 to 6, overall MARD and %20/20 agreement rates were 9.3% and 91.5%, respectively. In older children and adolescents aged 7 to 17 years, arm- and abdomen-placed G7 sensor MARD values were 8.1% and 9.0%, respectively, and overall %20/20 values were 95.3% and 92.9%, respectively. These results are consistent with those of the G6 system in children and adolescents with T1D and with an earlier study of the G5 system that evaluated accuracy at different insertion sites. Compared with the G6 RT-CGM system, the G7 has a shorter warm-up period (27 minutes vs 2 hours), making it possible for youth and caregivers of young children to obtain glucose data more quickly for diabetes management decisions. According to the authors, while day 1 accuracy tends to be lower across CGM devices, the accuracy of the G7—coupled with its shorter warm-up period—should improve the sensor experience in young users. The G7 RT-CGM was recently FDA approved for use in all individuals with diabetes aged 2 years and older, and having data specific to use in children and adolescents can be of value for managed care and payer professionals in developing coverage policies.
Laffel LM, Bailey TS, Christiansen MP, Reid JL, Beck SE. Accuracy of a Seventh-Generation Continuous Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. J Diabetes Sci Technol. 2022:19322968221091816.
Learn More

G7 Real-Time Continuous Glucose Monitoring System Demonstrates Accurate Glucose Readings and No Serious Adverse Events in Adults with Type 1 and Type 2 Diabetes
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
The accuracy and safety of the G7 real-time continuous glucose monitoring (RT-CGM) system was demonstrated over 10.5 days of use in adults with diabetes. In the study, adults with either type 1 or type 2 diabetes—either on intensive insulin therapy or not—wore G7 sensors concurrently on the upper arm and abdomen. Individuals were seen in clinic on days 1 or 2, 4 or 7, and on the second half of day 10 or the first half of day 11 for frequent comparisons with comparator blood glucose measurements obtained with the YSI 2300 Stat Plus glucose analyzer. After analyzing data from 316 participants (619 sensors, 77,774 matched pairs), overall mean absolute relative differences (MARD) between RT-CGM and YSI values for arm- and abdomen-placed sensors were 8.2% and 9.1%, respectively. In-clinic glucose manipulations and frequent blood glucose sampling confirmed accurate readings during euglycemia, hypoglycemia, and hyperglycemia (reflected as time in range [TIR], time below range [TBR], and time above range [TAR]), as well as during rapid glucose concentration change. Even at the highest rates of glucose concentration change, MARD values <10% were observed for arm-placed sensors and were <10.5% for abdomen-placed sensors. Offering a smaller size and added features, such as sensor/transmitter integration with a simplified insertion process, the G7 also demonstrated a favorable safety profile, with no serious adverse events reported during the study. With the recent FDA approval of the G7 RT-CGM system, payer professionals may well find value in the accuracy and safety data from this prospective multicenter single-arm study when they are developing coverage criteria.
Garg SK, et al. Accuracy and Safety of Dexcom G7 Continuous Glucose Monitoring in Adults with Diabetes. Diabetes Technol Ther. 2022;24(6):373-380.
Learn More

National Institute for Health and Care Excellence (NICE) Medtech Innovation Briefing for Dexcom G6 Real-Time CGM
November 3, 2020Economic Outcomes Article / Publication / CGM Innovations
Source:
Key Takeaway: The intended place in therapy is as an alternative to routine blood glucose monitoring in people (over 2 years old), including pregnant women, with type 1 or type 2 diabetes, who use multiple daily insulin injections or use insulin pumps and are self-managing their diabetes. Dexcom G6 could reduce costs and would benefit the healthcare system by improving long-term outcomes, reducing the need for intensive treatment and, in the short term, reducing severe hypoglycaemic events leading to hospital admissions. Remote care may reduce the need for hospital visits.
Learn More- 1
- 1-9 of 9 results
Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources


New G7 Continuous Glucose Monitoring System Demonstrates Similar Accuracy and Superior Ease-of-Use Compared with Previous Versions
In an analysis of available data, accuracy metrics from preapproval trials of the G5, G6, and G7 real-time continuous glucose monitoring (RT-CGM) systems were compared after propensity score adjustments were applied to balance baseline demographic characteristics. Metrics included mean absolute relative differences (MARD) between CGM and YSI values (from the YSI, Inc., 2300 Stat Plus system) and the proportion of CGM values within 20% or 20 mg/dL of the YSI values (“%20/20”). Ease-of-use was also evaluated by formal task analysis in the study. Accuracy performance of the G7 sensors, whether placed on the arm or abdomen, was similar to that of abdomen-placed G5 and G6 sensors, and egregious errors were rare with all three systems. Based on the formal task analysis, the authors concluded that simplification of the sensor insertion process should result in G7 being even easier to learn and several software improvements may contribute to better glycemic outcomes. Payer professionals may well find confidence in the comparative usability data for the latest RT-CGM system versus previous versions of the technology when considering updates to coverage policies.
Welsh JB, Psavko S, Zhang X, Gao P, Balo AK. Comparisons of Fifth-, Sixth-, and Seventh-Generation Continuous Glucose Monitoring Systems. J Diabetes Sci Technol. 2022:19322968221099879.
Learn More

In Children and Adolescents with Type 1 Diabetes, G7 Continuous Glucose Monitoring System Maintains Accuracy Across Wear Days, Glucose Ranges, and Rates of Glucose Change
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
In children and adolescents with type 1 diabetes (T1D), the G7 real-time continuous glucose monitoring (RT-CGM) system demonstrated accuracy across wear days, glucose ranges, and rates of glucose change. Over 10.5 days, RT-CGM data from 28 participants aged 2 to 6 years and 127 participants aged 7 to 17 were collected and compared with blood glucose measurements. In young children aged 2 to 6, overall MARD and %20/20 agreement rates were 9.3% and 91.5%, respectively. In older children and adolescents aged 7 to 17 years, arm- and abdomen-placed G7 sensor MARD values were 8.1% and 9.0%, respectively, and overall %20/20 values were 95.3% and 92.9%, respectively. These results are consistent with those of the G6 system in children and adolescents with T1D and with an earlier study of the G5 system that evaluated accuracy at different insertion sites. Compared with the G6 RT-CGM system, the G7 has a shorter warm-up period (27 minutes vs 2 hours), making it possible for youth and caregivers of young children to obtain glucose data more quickly for diabetes management decisions. According to the authors, while day 1 accuracy tends to be lower across CGM devices, the accuracy of the G7—coupled with its shorter warm-up period—should improve the sensor experience in young users. The G7 RT-CGM was recently FDA approved for use in all individuals with diabetes aged 2 years and older, and having data specific to use in children and adolescents can be of value for managed care and payer professionals in developing coverage policies.
Laffel LM, Bailey TS, Christiansen MP, Reid JL, Beck SE. Accuracy of a Seventh-Generation Continuous Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. J Diabetes Sci Technol. 2022:19322968221091816.
Learn More

G7 Real-Time Continuous Glucose Monitoring System Demonstrates Accurate Glucose Readings and No Serious Adverse Events in Adults with Type 1 and Type 2 Diabetes
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
The accuracy and safety of the G7 real-time continuous glucose monitoring (RT-CGM) system was demonstrated over 10.5 days of use in adults with diabetes. In the study, adults with either type 1 or type 2 diabetes—either on intensive insulin therapy or not—wore G7 sensors concurrently on the upper arm and abdomen. Individuals were seen in clinic on days 1 or 2, 4 or 7, and on the second half of day 10 or the first half of day 11 for frequent comparisons with comparator blood glucose measurements obtained with the YSI 2300 Stat Plus glucose analyzer. After analyzing data from 316 participants (619 sensors, 77,774 matched pairs), overall mean absolute relative differences (MARD) between RT-CGM and YSI values for arm- and abdomen-placed sensors were 8.2% and 9.1%, respectively. In-clinic glucose manipulations and frequent blood glucose sampling confirmed accurate readings during euglycemia, hypoglycemia, and hyperglycemia (reflected as time in range [TIR], time below range [TBR], and time above range [TAR]), as well as during rapid glucose concentration change. Even at the highest rates of glucose concentration change, MARD values <10% were observed for arm-placed sensors and were <10.5% for abdomen-placed sensors. Offering a smaller size and added features, such as sensor/transmitter integration with a simplified insertion process, the G7 also demonstrated a favorable safety profile, with no serious adverse events reported during the study. With the recent FDA approval of the G7 RT-CGM system, payer professionals may well find value in the accuracy and safety data from this prospective multicenter single-arm study when they are developing coverage criteria.
Garg SK, et al. Accuracy and Safety of Dexcom G7 Continuous Glucose Monitoring in Adults with Diabetes. Diabetes Technol Ther. 2022;24(6):373-380.
Learn More

National Institute for Health and Care Excellence (NICE) Medtech Innovation Briefing for Dexcom G6 Real-Time CGM
November 3, 2020Economic Outcomes Article / Publication / CGM Innovations
Source:
Key Takeaway: The intended place in therapy is as an alternative to routine blood glucose monitoring in people (over 2 years old), including pregnant women, with type 1 or type 2 diabetes, who use multiple daily insulin injections or use insulin pumps and are self-managing their diabetes. Dexcom G6 could reduce costs and would benefit the healthcare system by improving long-term outcomes, reducing the need for intensive treatment and, in the short term, reducing severe hypoglycaemic events leading to hospital admissions. Remote care may reduce the need for hospital visits.
Learn More- 1
- 1-9 of 9 results
Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources


In Children and Adolescents with Type 1 Diabetes, G7 Continuous Glucose Monitoring System Maintains Accuracy Across Wear Days, Glucose Ranges, and Rates of Glucose Change
In children and adolescents with type 1 diabetes (T1D), the G7 real-time continuous glucose monitoring (RT-CGM) system demonstrated accuracy across wear days, glucose ranges, and rates of glucose change. Over 10.5 days, RT-CGM data from 28 participants aged 2 to 6 years and 127 participants aged 7 to 17 were collected and compared with blood glucose measurements. In young children aged 2 to 6, overall MARD and %20/20 agreement rates were 9.3% and 91.5%, respectively. In older children and adolescents aged 7 to 17 years, arm- and abdomen-placed G7 sensor MARD values were 8.1% and 9.0%, respectively, and overall %20/20 values were 95.3% and 92.9%, respectively. These results are consistent with those of the G6 system in children and adolescents with T1D and with an earlier study of the G5 system that evaluated accuracy at different insertion sites. Compared with the G6 RT-CGM system, the G7 has a shorter warm-up period (27 minutes vs 2 hours), making it possible for youth and caregivers of young children to obtain glucose data more quickly for diabetes management decisions. According to the authors, while day 1 accuracy tends to be lower across CGM devices, the accuracy of the G7—coupled with its shorter warm-up period—should improve the sensor experience in young users. The G7 RT-CGM was recently FDA approved for use in all individuals with diabetes aged 2 years and older, and having data specific to use in children and adolescents can be of value for managed care and payer professionals in developing coverage policies.
Laffel LM, Bailey TS, Christiansen MP, Reid JL, Beck SE. Accuracy of a Seventh-Generation Continuous Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. J Diabetes Sci Technol. 2022:19322968221091816.
Learn More

G7 Real-Time Continuous Glucose Monitoring System Demonstrates Accurate Glucose Readings and No Serious Adverse Events in Adults with Type 1 and Type 2 Diabetes
December 9, 2022Clinical Outcomes Article / Publication / CGM Innovations
The accuracy and safety of the G7 real-time continuous glucose monitoring (RT-CGM) system was demonstrated over 10.5 days of use in adults with diabetes. In the study, adults with either type 1 or type 2 diabetes—either on intensive insulin therapy or not—wore G7 sensors concurrently on the upper arm and abdomen. Individuals were seen in clinic on days 1 or 2, 4 or 7, and on the second half of day 10 or the first half of day 11 for frequent comparisons with comparator blood glucose measurements obtained with the YSI 2300 Stat Plus glucose analyzer. After analyzing data from 316 participants (619 sensors, 77,774 matched pairs), overall mean absolute relative differences (MARD) between RT-CGM and YSI values for arm- and abdomen-placed sensors were 8.2% and 9.1%, respectively. In-clinic glucose manipulations and frequent blood glucose sampling confirmed accurate readings during euglycemia, hypoglycemia, and hyperglycemia (reflected as time in range [TIR], time below range [TBR], and time above range [TAR]), as well as during rapid glucose concentration change. Even at the highest rates of glucose concentration change, MARD values <10% were observed for arm-placed sensors and were <10.5% for abdomen-placed sensors. Offering a smaller size and added features, such as sensor/transmitter integration with a simplified insertion process, the G7 also demonstrated a favorable safety profile, with no serious adverse events reported during the study. With the recent FDA approval of the G7 RT-CGM system, payer professionals may well find value in the accuracy and safety data from this prospective multicenter single-arm study when they are developing coverage criteria.
Garg SK, et al. Accuracy and Safety of Dexcom G7 Continuous Glucose Monitoring in Adults with Diabetes. Diabetes Technol Ther. 2022;24(6):373-380.
Learn More

National Institute for Health and Care Excellence (NICE) Medtech Innovation Briefing for Dexcom G6 Real-Time CGM
November 3, 2020Economic Outcomes Article / Publication / CGM Innovations
Source:
Key Takeaway: The intended place in therapy is as an alternative to routine blood glucose monitoring in people (over 2 years old), including pregnant women, with type 1 or type 2 diabetes, who use multiple daily insulin injections or use insulin pumps and are self-managing their diabetes. Dexcom G6 could reduce costs and would benefit the healthcare system by improving long-term outcomes, reducing the need for intensive treatment and, in the short term, reducing severe hypoglycaemic events leading to hospital admissions. Remote care may reduce the need for hospital visits.
Learn More- 1
- 1-9 of 9 results
Sign Up To Stay Current On The Latest Coverage
Updates, Recent News, And Resources


G7 Real-Time Continuous Glucose Monitoring System Demonstrates Accurate Glucose Readings and No Serious Adverse Events in Adults with Type 1 and Type 2 Diabetes
The accuracy and safety of the G7 real-time continuous glucose monitoring (RT-CGM) system was demonstrated over 10.5 days of use in adults with diabetes. In the study, adults with either type 1 or type 2 diabetes—either on intensive insulin therapy or not—wore G7 sensors concurrently on the upper arm and abdomen. Individuals were seen in clinic on days 1 or 2, 4 or 7, and on the second half of day 10 or the first half of day 11 for frequent comparisons with comparator blood glucose measurements obtained with the YSI 2300 Stat Plus glucose analyzer. After analyzing data from 316 participants (619 sensors, 77,774 matched pairs), overall mean absolute relative differences (MARD) between RT-CGM and YSI values for arm- and abdomen-placed sensors were 8.2% and 9.1%, respectively. In-clinic glucose manipulations and frequent blood glucose sampling confirmed accurate readings during euglycemia, hypoglycemia, and hyperglycemia (reflected as time in range [TIR], time below range [TBR], and time above range [TAR]), as well as during rapid glucose concentration change. Even at the highest rates of glucose concentration change, MARD values <10% were observed for arm-placed sensors and were <10.5% for abdomen-placed sensors. Offering a smaller size and added features, such as sensor/transmitter integration with a simplified insertion process, the G7 also demonstrated a favorable safety profile, with no serious adverse events reported during the study. With the recent FDA approval of the G7 RT-CGM system, payer professionals may well find value in the accuracy and safety data from this prospective multicenter single-arm study when they are developing coverage criteria.
Garg SK, et al. Accuracy and Safety of Dexcom G7 Continuous Glucose Monitoring in Adults with Diabetes. Diabetes Technol Ther. 2022;24(6):373-380.
Learn More

National Institute for Health and Care Excellence (NICE) Medtech Innovation Briefing for Dexcom G6 Real-Time CGM
November 3, 2020Economic Outcomes Article / Publication / CGM Innovations
Source:
Key Takeaway: The intended place in therapy is as an alternative to routine blood glucose monitoring in people (over 2 years old), including pregnant women, with type 1 or type 2 diabetes, who use multiple daily insulin injections or use insulin pumps and are self-managing their diabetes. Dexcom G6 could reduce costs and would benefit the healthcare system by improving long-term outcomes, reducing the need for intensive treatment and, in the short term, reducing severe hypoglycaemic events leading to hospital admissions. Remote care may reduce the need for hospital visits.
Learn More- 1
- 1-9 of 9 results


National Institute for Health and Care Excellence (NICE) Medtech Innovation Briefing for Dexcom G6 Real-Time CGM
Source:
Key Takeaway: The intended place in therapy is as an alternative to routine blood glucose monitoring in people (over 2 years old), including pregnant women, with type 1 or type 2 diabetes, who use multiple daily insulin injections or use insulin pumps and are self-managing their diabetes. Dexcom G6 could reduce costs and would benefit the healthcare system by improving long-term outcomes, reducing the need for intensive treatment and, in the short term, reducing severe hypoglycaemic events leading to hospital admissions. Remote care may reduce the need for hospital visits.
Learn More- 1
- 1-9 of 9 results